Pfizer (PFE) Gets CHMP Recommendation for COVID Pill Paxlovid
Pfizer PFE announced that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has recommended the conditional marketing authorization (CMA) of its promising oral antiviral candidate for COVID-19, Paxlovid. A decision from the European Commission related to the CMA for Paxlovid is expected shortly.
The company is seeking CMA for Paxlovid (co-administration of nirmatrelvir and a low dose of ritonavir) to treat mild-to-moderate COVID-19 in adult patients who do not require supplemental oxygen and who are at increased risk of hospitalizations or death.
Following a potential approval from the EC, Paxlovid will be the first oral treatment available for treating COVID-19 in Europe.
The CHMP’s recommendation for Paxlovid is based on clinical data from an interim analysis of a phase II/III study, EPIC-HR study. Data from the study showed that Paxlovid reduced the risk of hospitalization or death by 89% in non-hospitalized adult patients with COVID-19 at high risk of progressing to severe illness compared to placebo within three days of symptom onset. Similar benefits were observed in patients treated within five days of symptom onset.
Paxlovid received emergency use authorization from the FDA last month based on data from the EPIC-HR study. The drug is currently authorized for emergency use or approved in more than 10 countries.
We note that Pfizer expects to manufacture 120 million courses of Paxlovid by the end of 2022 for the supply of the drug in countries across the globe where it has gained authorization/approval.
Meanwhile, Pfizer is also evaluating Paxlovid in a phase II/III study — EPIC-SR — efficacy and safety in adults with a confirmed diagnosis of SARS-CoV-2 infection who are at low risk of hospitalization or death.
Pfizer’s stock has risen 48.8% this year so far compared with an increase of 14.2% for the industry.
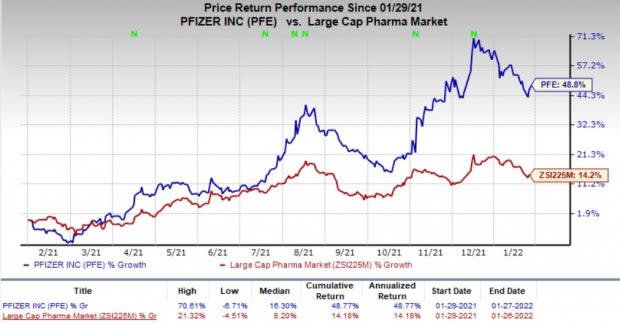
Image Source: Zacks Investment Research
Apart from Paxlovid, Merck MRK is another company with an oral COVID-19 drug — molnupiravir— authorized in the United States. Although Merck’s drug is not yet authorized in Europe, the EMA had issued an advice for the use of Merck’s molnupiravir to treat adults with COVID-19 who do not require supplemental oxygen and who are at increased risk of developing severe COVID-19 in November 2021. Per this EMA advice, national authorities may decide on the possible early use of the medicine prior to marketing authorization.
Merck expects molnupiravir’s global opportunity to be approximately $5 billion to $7 billion through 2022.
Apart from these two oral COVID-19 drugs, there are a few other authorized/approved intravenous options available to treat COVID-19. Gilead GILD was the first company to bring a treatment for COVID-19 infections — Veklury — for patients who require hospitalization. Gilead’s drug is also the only COVID-19 drug to have received “full” approval from the FDA.
Earlier this month, the FDA approved the label expansion of Gilead’s antiviral treatment Veklury to include treatment of non-hospitalized COVID patients who are at high risk of progression to severe COVID-19, including hospitalization or death.
Earlier this week, the FDA revised EUA for two cocktail antibody drugs — Eli Lilly’s LLY bamlanivimab plus etesevimab, and Regeneron Pharmaceuticals’ REGEN-COV (casirivimab plus imdevimab). The revision for Lilly and Regeneron’s cocktail COVID-19 drugs limits their use in patients who have been infected or exposed to a coronavirus variant that is susceptible to these treatments.
The FDA stated that Eli Lilly and Regeneron’s COVID-19 drugs are currently not authorized for use in any U.S. states, territories, and jurisdictions. The COVID-19 Treatment Guidelines Panel of the National Institute of Health (“NIH”) also recently recommended against the use of Eli Lilly and Regeneron’s COVID-19 drugs due to their markedly reduced activity against the Omicron variant.
Lilly and Regeneron’s cocktail drugs were among the few treatment options available till late 2021 for COVID-19 infections. These two drugs generated sales worth a few billion dollars in aggregate in the first nine months.
The unavailability of the two popular COVID-19 drugs may boost the demand for the existing COVID-19 treatment options, especially Pfizer and Merck’s oral drugs as they are easy to administer.
Pfizer Inc. Price
Pfizer Inc. price | Pfizer Inc. Quote
Zacks Rank
Pfizer currently has a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Merck & Co., Inc. (MRK) : Free Stock Analysis Report
Eli Lilly and Company (LLY) : Free Stock Analysis Report
Gilead Sciences, Inc. (GILD) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 