Lilly (LLY) Hits Record High on Crohn's Disease Study Data
Shares of Eli Lilly LLY rose nearly 3% on Tuesday after it reported detailed one-year results from the phase III VIVID-1 study, which evaluated its IL-23p19 antibody mirikizumab in adults with Crohn’s disease (CD).
While management had previously reported that the study VIVID-1 study achieved its co-primary and all major secondary endpoints, the latest press release showed that mirikizumab also worked in bio-failed patients (harder-to-treat patients for whom biologics either did not or stopped working).
Per Lilly, 39.3% of bio-naïve patients (those who had never tried a biologic) who received mirikizumab across a 52-week treatment period achieved an endoscopic response compared with 11.8% for those patients on placebo. With regard to bio-failed patients, 36.7% of patients on mirikizumab achieved endoscopic response compared with 6.2% on placebo.
Further, 47.3% of bio-naïve and 43.4% of bio-failed patients on mirikizumab achieved clinical remission at week 52 compared with 26.5% and 12.4% of patients on placebo, respectively.
Per management, the response rates and treatment effects were observed across both groups. Overall data from the study showed that 54.1% of patients treated with mirikizumab achieved clinical remission, while 48.4% achieved an endoscopic response following one year of treatment.
However, the results were mixed when the results when the Lilly drug was pitted against J&J’s JNJ blockbuster drug Stelara (ustekinumab) in CD indication. While patients taking mirikizumab achieved a combined 52-week clinical remission and endoscopic response, which were ‘numerically higher’ than the J&J drug, it wasn’t able to achieve superiority to Stelara in the endoscopic response endpoint.
Following the results announcement, shares of Lilly hit an all-time high price of $803.17. Wall Street has been paying a lot of attention to the stock thanks to the impressive sales performance of its obesity drug. With the latest results, investors were likely impressed as the company is not just limiting itself to one segment but also exploring other medication areas like immunology, which have shown immense commercial potential over the years.
Eli Lilly’s shares have surged 37.8% year to date compared with the industry’s 16.8% growth.
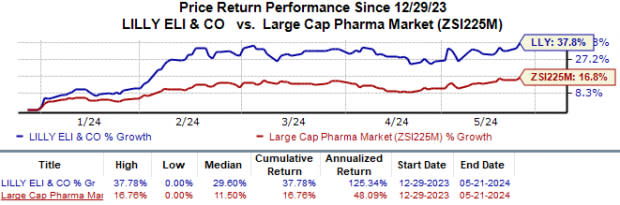
Image Source: Zacks Investment Research
Mirikizumab is currently approved in the United States for treating ulcerative colitis (UC) under the brand name Omvoh. This approval was secured by Lilly last year in October.
Ahead of this data release, Lilly has submitted regulatory filings with the FDA and EMA seeking approval for Omvoh in CD indication, with data supported by the VIVID-1 study.
The targeted CD markets are highly competitive. If approved, Lilly will face stiff competition from AbbVie ABBV, which markets its own drug Skyrizi, which utilizes a similar mechanism of action to treat CD. A blockbuster IL-23 inhibitor, AbbVie’s Skyrizi received label expansion in the United States and Europe in CD indication in 2022.
Last year, ABBV reported data from a head-to-head phase III study that compared Skyrizi to J&J’s Stelara in CD indication. This study achieved all its primary and secondary endpoints, thereby demonstrating the superiority of the AbbVie drug over J&J’s.
Lilly is also exploring therapeutics in other segments beyond cardiovascular and immunology. Earlier this week, it inked a deal with privately-held Aktis Oncology to develop novel targeted radiopharmaceuticals to treat a broad range of solid tumors. Per the agreement terms, Lilly will make an upfront payment of $60 million to Aktis in addition to an equity investment by Lilly. Aktis will also be eligible to receive milestone payments of up to $1.1 billion and potential royalties on sales.
Eli Lilly and Company Price
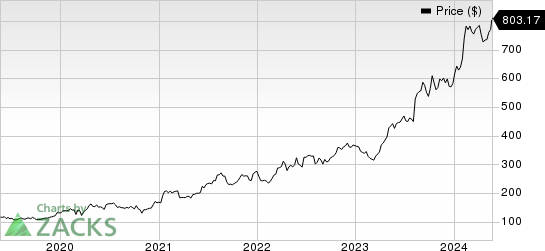
Eli Lilly and Company price | Eli Lilly and Company Quote
Zacks Rank
Eli Lilly currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
Eli Lilly and Company (LLY) : Free Stock Analysis Report
AbbVie Inc. (ABBV) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 