GSK's RSV Vaccine Arexvy Gets FDA Nod for Adults 50-59 Years
GSK GSK announced that the FDA has approved the expanded use of its respiratory syncytial virus (RSV) vaccine, Arexvy for adults aged 50-59 who are at increased risk of RSV disease. Arexvy is currently approved in the United States, Europe, Japan and several other countries in adults aged 60 and more for the prevention of lower respiratory tract disease (LRTD) caused by RSV. With the latest approval, Arexvy becomes the first RSV vaccine approved for adults aged 50-59 who are at high risk.
In the United States, there are over 13 million adults aged 50-59 who are at an increased risk for RSV disease as they have an underlying medical condition, such as chronic obstructive pulmonary disease, asthma, heart failure and diabetes and will benefit from this vaccine.
The approval was based on data from a phase III study, which showed that immune responses were non-inferior in adults aged 50-59 at increased risk for RSV disease compared to adults aged 60 and older.
A regulatory application seeking label expansion of Arexvy for adults aged 50-59 is also under review in the EU, Japan and some other countries. Clinical studies on Arexvy for expanded use in adults aged 18-49 are also ongoing with data read-outs expected in the second half of the year.
GSK’s stock has risen 11.2% so far this year against a decline of 5.2% for the industry.
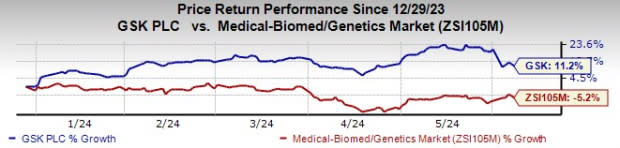
Image Source: Zacks Investment Research
Arexvy was the first RSV vaccine for older adults to be approved anywhere in the world. Arexvy had an exceptional launch and generated £1.2 billion in sales in 2023. In the first quarter of 2024, Arexvy generated £182 million in sales. Though sales fell significantly when compared with fourth-quarter 2023 sales of £529 million, the downside was in line with anticipated seasonality patterns. Arexvy sales are expected to be strong in 2024, driven by further penetration in the U.S. market as well as early adoption from international launches. Over time, GSK expects Arexvy to generate more than £3 billion in annual sales.
Pfizer’s PFE RSV vaccine, Abrysvo, was launched to help protect older adults as well as infants through maternal immunization in the United States as well as in the EU in 2023. Abrysvo recorded sales of $890 million in 2023.
In April this year, Pfizer announced that a phase III study evaluating its Abrysvo in adults aged 18 to 59 met its primary endpoints. Pfizer plans to seek approval from the FDA for the expanded use of Abrysvo for adults 18 years and older by submitting these data. However, the company has not provided any timeline for the filing.
Moderna MRNA has filed regulatory applications for its mRNA vaccine, mRNA-1345, for the prevention of RSV-LRTD and acute respiratory disease in adults over 60 years of age. It has also initiated multiple phase III studies of its RSV vaccine in adults over 50 years of age to evaluate co-administration and revaccination. Moderna is also evaluating mRNA-1345 in early-stage studies for high-risk adults, as well as maternal and pediatric populations.
AstraZeneca AZN and Sanofi’s RSV antibody called Beyfortus was approved in Europe for protection against LRTI caused by RSV in newborns and infants in 2022 and in the United States in 2023. Sanofi leads the commercialization activities and records revenues on Beyfortus. AstraZeneca leads the manufacturing activities and records a 50% share of gross profits on sales of Beyfortus in major markets outside the United States received from Sanofi. AstraZeneca also records Beyfortus product sales from products supplied to partner Sanofi.
GSK currently has a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
GSK PLC Sponsored ADR Price and Consensus
GSK PLC Sponsored ADR price-consensus-chart | GSK PLC Sponsored ADR Quote
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
AstraZeneca PLC (AZN) : Free Stock Analysis Report
GSK PLC Sponsored ADR (GSK) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Moderna, Inc. (MRNA) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 