Gamida Cell (GMDA) Surges on FDA Approval for Omisirge
Gamida Cell GMDA surged almost 39% on Apr 17, after receiving FDA approval for Omisirge (Omidubicel), a stem cell therapy for patients with hematologic malignancies (blood cancers). The therapy involvesumbilical cord blood transplantation following myeloablative conditioning to reduce the time to neutrophil recovery and the incidence of infection.
The approval of Omisirge, the first approved product of GMDA, will significantly boost the company’s growth prospects.
The allogeneic cell therapy was earlier granted Breakthrough Therapy Designation and priority review by the FDA. It has also received Orphan Drug Designation in EU and the United States.
This FDA approval marks a significant advancement in treating patients with blood cancers. The abovementioned therapy can potentially increase access to stem cell transplantation, particularly for patients from ethnically diverse backgrounds who struggle to find a matched donor.
Gamida Cell's shares have plunged 64.6% in the past year compared with the industry's 11.4% decline.
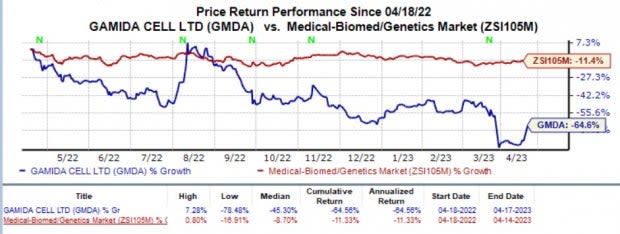
Image Source: Zacks Investment Research
The company is looking for strategic partnerships to support the launch and commercialization of Omisirge in the United States and the rest of the world. The therapy is currently available in the country for transplant centers.
GMDA recently announced a strategic reconstruction to focus on the launch of Omisirge. The company intends to reduce its headcount by 17%, with most of the terminations tied to discontinuation of the pre-clinical N.K. cell therapy candidates. It has also closed some of its manufacturing facilities.
Total cash and cash equivalents were $64.7 million as of Dec 30, 2022. GMDA expects to extend its cash runway through the third quarter of 2023.
The FDA approval was based on the randomized phase III clinical study results, where Omisirge demonstrated faster neutrophil recovery, and reduced bacterial and fungal infections compared to standard cord blood.
Omidubicel is also being evaluated in an ongoing investigator-sponsored early-stage study for patients with severe aplastic anemia. GDA-201 is the company's other early-stage immunotherapy candidate, evaluated for treating hematologic and solid tumors.
Gamida Cell Ltd. Price and Consensus
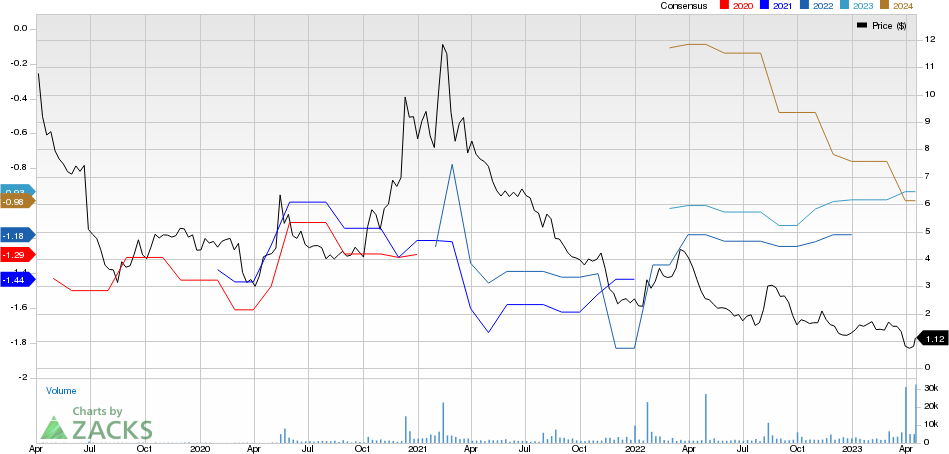
Gamida Cell Ltd. price-consensus-chart | Gamida Cell Ltd. Quote
Zacks Rank & Stocks to Consider
Currently, Gamida Cell has a Zacks Rank #3 (Hold).
Some better-ranked stocks for investors interested in the same sector are CRISPR Therapeutics CRSP, Kala Pharmaceuticals KALA and Allogene Therapeutics ALLO, each carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today's Zacks #1 Rank (Strong Buy) stocks here.
Loss per share estimates for CRISPR Therapeutics have narrowed from $8.21 to $7.35 for 2023 in the past 60 days.
CRSP's earnings beat estimates in two of the trailing four quarters and missed the mark in the other two, the average surprise being 3.19%. The company's shares have plunged 11.4% in the past year.
Loss per share estimates for Kala Pharmaceuticals have narrowed from $18.34 to $16.54 for 2023 and from $14.41 to $13.12 for 2024 in the past 60 days. KALA's shares have plunged 57.7% in the past year.
The company's earnings beat estimates in two of the last four quarters and missed the mark in the other two, the average surprise being 11.56%.
Loss per share estimates for Allogene Therapeutics have narrowed from $2.83 to $2.44 for 2023 and from $2.69 to $2.46 for 2024 in the past 60 days.
ALLO's earnings beat estimates in each of the trailing four quarters, the average surprise being 8.33%. The company's shares have plunged 37.8% in the past year.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
CRISPR Therapeutics AG (CRSP) : Free Stock Analysis Report
Kala Pharmaceuticals, Inc. (KALA) : Free Stock Analysis Report
Allogene Therapeutics, Inc. (ALLO) : Free Stock Analysis Report
Gamida Cell Ltd. (GMDA) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 