Coronavirus update: 'Game-changer' Abbott test gets boost from Trump as Moderna shows vaccine progress
The Trump administration announced a $760 million deal with Abbott Laboratories (ABT) for a new rapid COVID-19 antigen test, giving new impetus to the push to reopen schools and workplaces.
Testing for COVID-19 continues to be a bumpy ride in the U.S., with backlogs still occasionally reported in pockets of the U.S. despite regular emergency use authorizations from the U.S. Food and Drug Administration (FDA).
In the past 24 hours, the FDA has authorized two new tests — a saliva test from California-based Fluidigm (FLDM) and the $5 rapid antigen test from Abbott. The latter has been hailed as a game-changer, as it provides accurate and rapid results, as well as includes an app that will host shareable results. On Thursday, the U.S. Health and Human Services Department (HHS) and Department of Defense (DoD) said they will purchase 150 million tests.
The technology used is similar to at-home pregnancy tests, according to Abbott divisional vice president of Applied Research and Technology, Dr. John Hackett — except that it still requires a clinician to administer the test.
But a key reason it has spurred enthusiasm is it is the “only antigen test in the US that doesn’t require instrumentation,” Hackett said.
Almost all other tests require some sort of heavy or portable machine, including Abbott’s own IDNow rapid PCR test. The new test, BinaxNOW, is a handheld device and includes the necessary reagents to perform the test, which takes only 15 minutes to provide results.
The administration has previously purchased other Abbott tests as part of efforts to ramp up availability parts of the country, as well as add to inventory in the national stockpile. The company is ramping up manufacturing to ship tens of millions of the antigen tests by September and produce at least 50 million a month by October.
The news arrives at a time when schools in certain regions are moving to hold in-person classes at least part time, and businesses are trying to lure employees out of their work-from-home mode. Business Insider reported on Thursday that Wall Street giant Blackstone is aiming for most of its workers to return to the office after Labor Day, and plans to send them at-home COVID-19 tests to facilitate the transition.
Abbott is targeting schools and workplaces with the new test, saying the “health pass” for negatives tests— which is valid for seven days— can help move people through lines quicker to enter buildings. The antigen test is generally seen as accurate for positive results, but negative results can sometimes require another type of test to confirm.
The speed and convenience of the test can help boost access to testing, which is still strained during surges.
Recently, in order to accommodate spikes in some areas, major labs took steps to limit testing after a major backlog built up — repeating the struggle the companies faced at the start of the pandemic. Though it only took a week or two two clear out, rather than months, the move highlights the ongoing struggle in testing capacity.
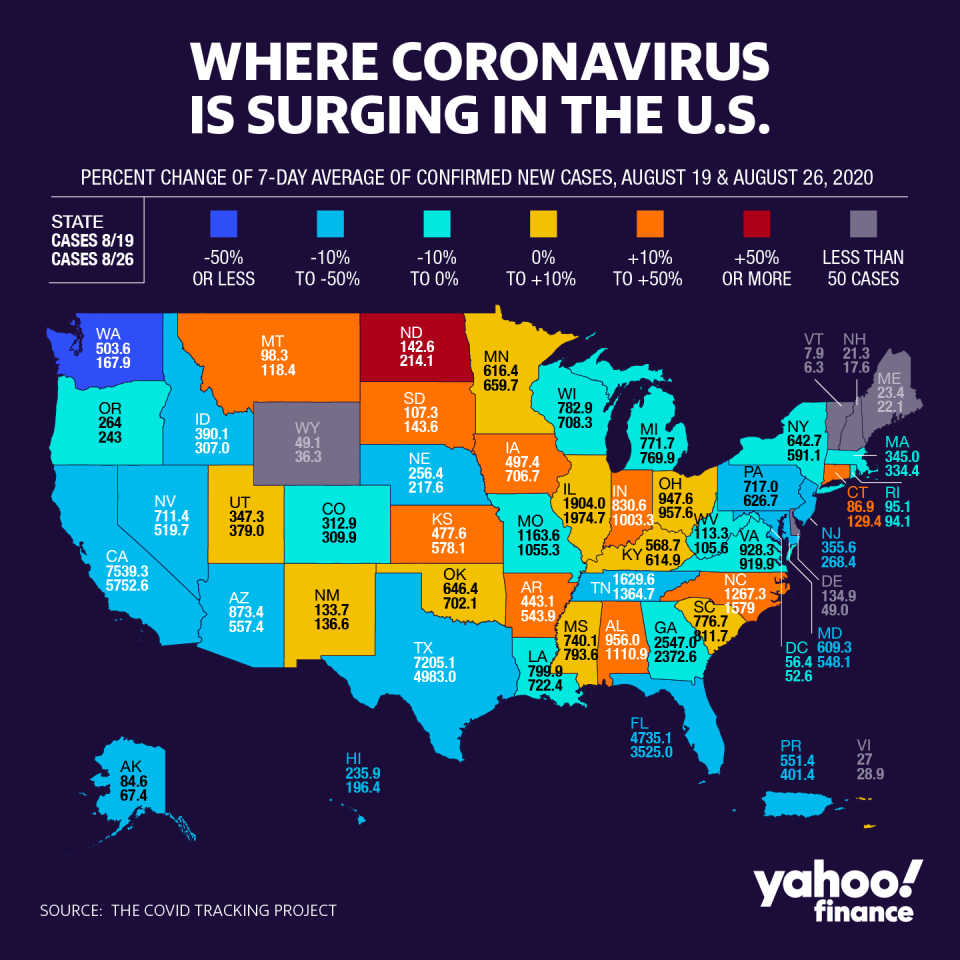
Separately, the Centers for Disease Control and Prevention (CDC) moved to clarify recently announced changes to testing guidelines. In a controversial move, the agency appeared to downplay the need for testing asymptomatic individuals from testing, which critics blasted as a political maneuver to hide positive cases— something President Donald Trump has previously hinted at.
In a media call Wednesday, White House coronavirus task force member Admiral Brett Giroir said the move was not meant to stop testing asymptomatic people altogether, but rather only in hot zones, as needed.
However, the Infectious Disease Society of America said in a statement that the move was “concerning” and called for a reversal as cases worldwide continue to rise, with symptom-less spreading a key driver. The pandemic has now affected more than 24 million, and killed more than 827,000. In the U.S., cases have surpassed 5.8 million and more than 180,000 Americans have died.
Vaccines on the horizon
As hopes build over a successful vaccine, Moderna (MRNA) and Pfizer (PFE) both presented data from their clinical trials to an advisory group to the CDC Wednesday.
New insight into Moderna’s data revealed that older adults responded similarly to the vaccine as the younger population. Those older than 65, and in some cases as old as in their 90s, produced similar neutralizing antibody responses, according to Moderna.
In a subsequent media call, executives said based on the positive news, the vaccine is likely to beat the FDA’s bar of 50%, possibly reaching 60% effectiveness.
A new center for infectious diseases
The National Institute of Allergy and Infectious Diseases has created a new center to study emerging infectious diseases that will include collaborations with 28 other countries.
The new channel of research mirrors a collaborative effort traditionally seen at the World Health Organization. Health experts are encouraged by the NIAID’s leadership, in the hopes the data will be less politically influenced
In a statement Thursday, NIAID announced 11 first-year grants totaling $17 million for the domestic and global research entities that will form the Centers for Research in Emerging Infectious Diseases (CREID), with at least $82 million committed over five years.
One of those principal investigators, Nikos Vasilakis, a professor and vice chair for research at The University of Texas Medical Branch, told Yahoo Finance the newfound collaboration of centers will carry “the full power, reputation and expertise” of the National Institutes of Health, as the funder, while improving on current practices through the collaborative effort.
The new partnership is based on equality, inclusion and partnerships in true meaning of those words, Vasilakis said.
In the past, organizations have acted independently, with some researchers “helicoptering in” to take samples, investigate, and then return to home institutions— without credit to those working on the ground in infectious zones. That will all change now, Vasilakis said.
“This new program is a great beginning and new basis for international partnerships and cooperation in this real threat we are facing. Especially now...we will start seeing a lot more emergent events of nasty viruses that affect” the world, Vasilakis said.
And leading the new effort is a familiar face, thanks to the current pandemic.
That is NIAID Director Anthony Fauci, who said in a statement.“The CREID network will enable early warnings of emerging diseases wherever they occur, which will be critical to rapid responses. The knowledge gained through this research will increase our preparedness for future outbreaks,”
Various centers will focus on different regions of the world, including Central and South America, Asia, and the Middle East. A coordinating center has been created in collaboration with RTI International in North Carolina’s Research Triangle Park.
Anjalee Khemlani is a reporter at Yahoo Finance. Follow her on Twitter: @AnjKhem
More from Anjalee:
Fauci: WHO 'imperfect but important' as coronavirus controversies batter agency
FL teacher explains why she retired because of coronavirus, doubts safe return to schools
How protests spurred Corporate America into action on race, inequality
Read the latest financial and business news from Yahoo Finance
Follow Yahoo Finance on Twitter, Facebook, Instagram, Flipboard, SmartNews, LinkedIn, YouTube.

 Yahoo Finance
Yahoo Finance 
