Catalyst Pharma's (CPRX) sNDA for Firdapse Approved by the FDA
Catalyst Pharmaceuticals CPRX announced that the FDA approved its supplemental new drug application (sNDA) for Firdapse (amifampridine) tablets in 10 mg dosage to include pediatric patients (six years and older) for treating Lambert-Eaton myasthenic syndrome (“LEMS”).
The company submitted an sNDA to the FDA for the use of Firdapse in treating pediatric LEMS patients in the first quarter of 2022.
LEMS is an ultra-rare disease autoimmune disorder characterized by muscle weakness of the limbs.
Post the sNDA approval by the FDA, Firdapse is now a treatment option in the United States for all LEMS patients beyond six years of age.
Shares of Catalyst have returned 87.6% against the industry’s decline of 28.4% in the year-to-date period.
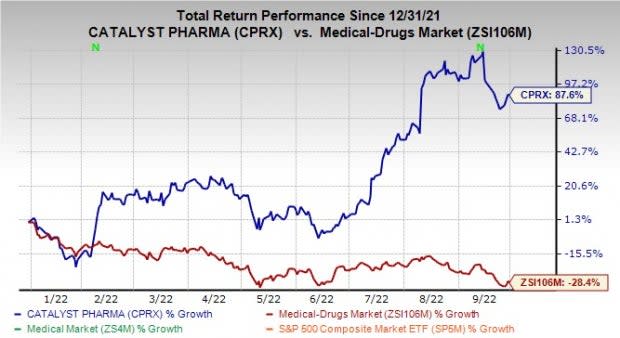
Image Source: Zacks Investment Research
Fridapse is already approved in the United States and the European Union for treating adults with LEMS. It is the only FDA-approved drug for LEMS in the United States.
Additionally, Firdapse enjoys a Breakthrough Therapy status and an Orphan Drug status from the FDA for treating LEMS.
Catalyst entered into a license agreement with DyDo Pharma in December 2021, to develop and commercialize Firdapse in Japan. To obtain regulatory approval for Firdapse from the Japanese regulatory authorities, Dydo has already initiated a phase III study evaluating the drug for LEMS in Japan.
Investors are reminded that Catalyst reached a settlement with Jacobus Pharamceuticals and PANTHERx RARE LLC on its patent infringement litigation in July 2022. The settlement marked the end of an ongoing dispute between Catalyst and Jacobus since the latter’s drug Ruzurgi (amifampridine) was approved by the FDA in 2019 for treating LEMS in pediatric patients. This was contested by CPRX in its lawsuit, claiming that the regulatory body’s approval violated the orphan drug designation granted to Firdapse for LEMS.
According to the settlement conditions, Catalyst will write off all claims related to the litigation between the companies.
Catalyst also acquired Jacobus’ intellectual property rights, which include the rights to develop and commercialize Ruzurgi in the United States and Mexico. In return, Catalyst will make a cash payment to Jacobus. Jacobus will also be eligible to receive low single-digit royalties on U.S. net sales of amifampridine.
However, Catalyst’s overdependence on Firdapse and the lack of any promising candidate in its pipeline can constrain its growth.
Catalyst Pharmaceuticals, Inc. Price
Catalyst Pharmaceuticals, Inc. price | Catalyst Pharmaceuticals, Inc. Quote
Zacks Rank and Stocks to Consider
Catalyst currently has a Zacks Rank #3 (Hold).
Some better-ranked stock in the same sector is Acer Therapeutics ACER, Aerie Pharmaceuticals AERI and Soleno Therapeutics SLNO, each carrying a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 (Strong Buy) Rank stocks here.
Acer Therapeutics’ loss per share estimates for 2022 remained steady at $2.47 in the past 30 days. The same for 2023 has remained steady at $1.07 during the same time frame.
Earnings of Acer missed estimates in two of the trailing four quarters and beat the same on the remaining two occasions. The average negative earnings surprise for ACER is 106.16%.
Aerie Therapeutics’ loss per share estimates for 2022 remained steady at $1.82 over the past 30 days. The same for 2023 has remained steady at 96 cents over the same time frame.
Earnings of Aerie beat estimates in three of the trailing four quarters and missed the same in the remaining occasion. The average earnings surprise for AERI is 70.27%.
Soleno Therapeutics’ loss per share estimates for 2022 remained steady at $4.05 over the past 30 days. The same for 2023 has remained steady at $2.10 in the same time frame.
Earnings of Soleno missed estimates in three of the trailing four quarters and were in line on the remaining occasion. The average earnings surprise for SLNO is 11.40%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Catalyst Pharmaceuticals, Inc. (CPRX) : Free Stock Analysis Report
Aerie Pharmaceuticals, Inc. (AERI) : Free Stock Analysis Report
Soleno Therapeutics, Inc. (SLNO) : Free Stock Analysis Report
Acer Therapeutics Inc. (ACER) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 