Biogen, Ionis shares rally as muscle drug for infants hits its goal
Shares of Biogen (BIIB) and Ionis Pharmaceuticals (IONS) soared Monday after an interim analysis of late-stage data showed their drug for a deadly muscle disorder in infants met its main goal.
Biogen stock rose 4.1 percent, while Ionis surged more than 30 percent.
The product, known as nusinersen, was developed by Biogen in collaboration with Ionis to treat spinal muscular atrophy, a genetic disease affecting the part of the nervous system that controls voluntary muscle movement. The disease can take away the ability to walk, eat, or breathe, and it is the leading cause of infant mortality, the companies said.
In the analysis, infants with SMA who got the drug achieved motor-skills development milestones better than those who didn't get the treatment, Biogen and Ionis said in a joint news release. The drug was also found to have an "acceptable" safety profile.
Based on the results, Biogen will stop the trial and all the patients will now receive the drug in a follow-on study, the companies said.
Biogen plans to develop and commercialize the drug globally. The company paid Ionis a $75 million licensing fee, and said it will, in the coming months, file to get the therapy approved by regulators.
Ionis is eligible to receive tiered royalties on any potential sales of nusinersen up to a certain percentage, and up to $150 million in milestone payments, according to Reuters.
"We remain committed to understanding the potential of nusinersen in the broader SMA population and will continue to focus on the rapid completion of our ongoing studies," B. Lynne Parshall, chief operating officer of Ionis, said in the same release.
Biogen's stock has dropped somewhat this year, falling more than 5 percent at one time.
BIIB 2016 Stock

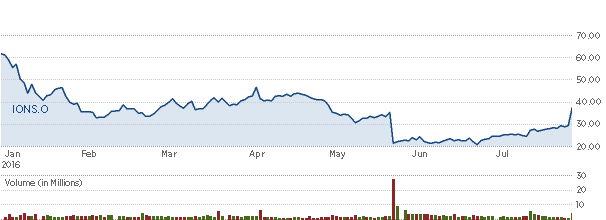
Ionis' stock has plummeted so far in 2016, at one point down more than 52 percent.
IONS 2016 Stock
More From CNBC
Top News and Analysis
Latest News Video
Personal Finance

 Yahoo Finance
Yahoo Finance 
