Acadia (ACAD) Gets FDA Approval for Daybue for Rett Syndrome
Acadia Pharmaceuticals ACAD announced that the FDA approved trofinetide for the treatment of Rett syndrome in adult and pediatric patients two years of age and older on Mar 10, 2023. The drug will be marketed in the United States under the brand name, Daybue. Per the company, Daybue currently stands as the first and only drug approved for the treatment of Rett syndrome.
In the past year, the shares of Acadia have witnessed a fall of 8.6% compared with the industry’s 11.2% decline.
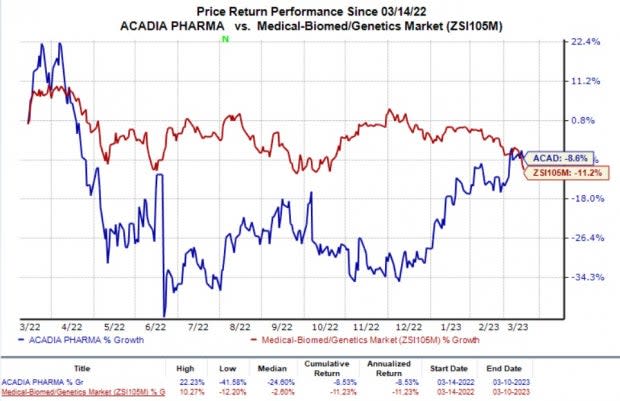
Image Source: Zacks Investment Research
The approval is based on the positive and statistically significant results from the company’s phase III LAVENDER study, which evaluated trofinetide’s efficacy and safety in 187 female patients with Rett syndrome from 5 to 20 years of age against treatment with placebo.
The study met both its co-primary endpoints, showing statistically significant improvements in treatment with Daybue against placebo, as measured by the change from baseline in Rett Syndrome Behaviour Questionnaire (RSBQ) total score (p=0.018) and the Clinical Global Impression-Improvement (CGI-I) scale score (p=0.003) at week 12. The RSBQ is a caregiver assessment that evaluates a range of symptoms pertaining to Rett syndrome, while the CGI-I is a global physician assessment of the improvement or worsening of patient condition.
Daybue (trofinetide) is Acadia’s synthetic version of a naturally occurring molecule known as the tripeptide glycine-proline-glutamate.
Rett syndrome is a rare, complex genetic neurodevelopmentaldisorder that may occur over four stages, affecting approximately 6,000-9,000 patients in the United States annually. Currently, approximately 4,500 patients are diagnosed with Rett syndrome according to an analysis of healthcare claims data. Rett syndrome is characterized by progressive loss of motor skills and language and it primarily affects females.
Acadia expects to make Daybue available in the United States by the end of April 2023. Additionally, per the terms of the company’s 2018 exclusive license agreement with Neuren Pharmaceuticals, the latter is entitled to develop and commercialize trofinetide in North America, for the treatment of Rett syndrome and other indications.
Acadia also received a Rare Pediatric Disease Priority Review Voucher from the FDA, along with the approval of Daybue, which can be used to obtain priority review for a subsequent application.
Another player, in the market currently conducting studies on possible treatment candidates for Rett syndrome, includes Anavex Life Sciences Corp. AVXL. Anavex is currently evaluating Anavex 2-73 (blarcamesine), a small-molecule activator of the sigma-1 receptor, for the treatment of Rett syndrome in a phase II/IIIEXCELLENCE (Anavex 2-73-RS-003) study, among other neurological indications.
Last month, Anavex announced the completion of enrollment of 92 patients in its phase II/III EXCELLENCE study for the treatment of Rett Syndrome in patients aged 5 to 17 years. The company anticipates announcing top-line data from the study in the second half of 2023.
ACADIA Pharmaceuticals Inc. Price and Consensus
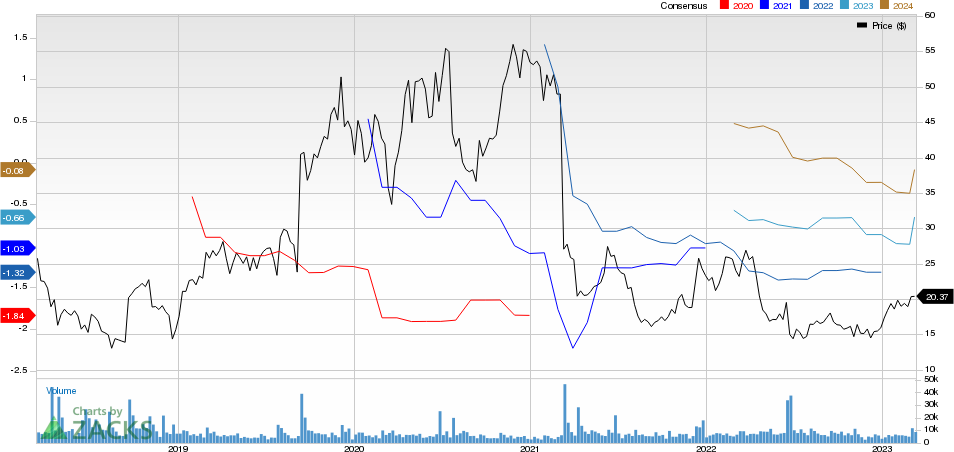
ACADIA Pharmaceuticals Inc. price-consensus-chart | ACADIA Pharmaceuticals Inc. Quote
Zacks Rank and Stocks to Consider
Acadia currently has a Zacks Rank #3 (Hold).
A couple of better-ranked stocks in the same industry are Aptinyx APTX and Annovis Bio ANVS, both carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 90 days, the estimate for Aptinyx’s 2023 loss per share has narrowed from 76 cents to 59 cents. In the past year, shares of Aptinyx have fallen 94.5%.
APTX’s earnings witnessed an average earnings surprise of 9.53%, beating all four estimates in the trailing four reported quarters.
In the past 90 days, the consensus estimate for Annovis’ 2023 loss per share has widened from $2.94 to $3.87. In the past year, shares of Annovis have increased by 17%.
ANVS’ earnings beat estimates in the last reported quarter, delivering an earnings surprise of 20.51%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
ACADIA Pharmaceuticals Inc. (ACAD) : Free Stock Analysis Report
Anavex Life Sciences Corp. (AVXL) : Free Stock Analysis Report
Aptinyx Inc. (APTX) : Free Stock Analysis Report
Annovis Bio, Inc. (ANVS) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 