Viking (VKTX) Falls 10% Despite Upbeat NASH Study Results
Viking Therapeutics VKTX announced positive 52-week histologic data from the phase IIb VOYAGE study that evaluated VK2809 in patients with biopsy-confirmed non-alcoholic steatohepatitis (NASH).
The VOYAGE study achieved its secondary endpoints evaluating histologic changes assessed by hepatic biopsy after 52 weeks of treatment with VK2809, when compared with placebo. This study evaluated four different doses of VK2809 — 1 mg, 2.5 mg, 5 mg and 10mg.
A significant portion of patients — ranging from 63% to 75% — who took VK2809 reached NASH resolution (meaning the disease symptoms disappeared) while experiencing no worsening of fibrosis. In contrast, only 29% of patients who received a placebo achieved similar results. Patients ranging from 44% to 57% who took the drug showed at least a one-stage improvement in fibrosis without worsening of NASH compared with 34% for placebo.
Overall, 40% to 50% of patients who received VK2809 achieved NASH resolution and at least a one-stage improvement in fibrosis compared with 20% in the placebo group.
We remind investors that the VOYAGE study has already achieved its primary endpoint – NASH patients who received VK2809 achieved a statistically significant reduction in liver fat content following 12 weeks of treatment.
Despite the positive study results, shares of Viking Therapeutics fell 9.7% on Jun 4. Before the opening bell, management held a conference call to discuss the VOYAGE study results. However, the company executives declined to provide any information on their plans for the drug and opted to wait until they discussed the results with the FDA. This did not sit well with investors who had been looking forward to Viking’s plans for late-stage development of the drug and plans for seeking regulatory approval for the drug.
Year to date, Viking Therapeutics’ shares have skyrocketed 202.2% against the industry’s 5.6% fall.
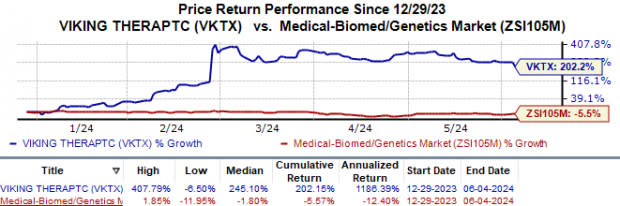
Image Source: Zacks Investment Research
Results from the VOYAGE study place Viking Therapeutics as a strong contender to Madrigal Pharmaceuticals MDGL, which recently received the FDA’s approval for the first-ever NASH drug. Madrigal’s NASH drug Rezdiffra was granted approval by the FDA under the accelerated pathway in March to treat NASH patients with moderate-to-advanced liver fibrosis. The drug was commercially launched by Madrigal in April.
Also called metabolic dysfunction-associated steatohepatitis (MASH), NASH is a progressive form of non-alcoholic fatty liver disease characterized by excessive fat buildup in the liver, accompanied by inflammation and fibrosis, which may progress to cirrhosis, liver failure, cancer and death.
Apart from Viking Therapeutics, several other companies like Akero Therapeutics AKRO and Ionis Pharmaceuticals IONS are also trying to develop a successful treatment for NASH indication.
Akero Therapeutics is evaluating its lead candidate, efruxifermin (EFX), for treating MASH across multiple clinical studies. In March, Akero reported updated results from the mid-stage HARMONY study evaluating EFX in pre-cirrhotic MASH patients. Patients who received the Akero drug achieved at least one-stage improvement in fibrosis with no worsening of MASH after 96 weeks of treatment for both the 50mg EFX (75%) and 28mg EFX (46%) dose groups compared with 24% for the placebo arm. The company is also evaluating the drug in the phase III SYNCHRONY program, which consists of three late-stage clinical studies. While two of the studies were initiated toward the end of 2023, the third one is on track to start later this year.
In March, Ionis reported positive results from a phase II study on its investigational drug ION224 in adult patients with MASH over a 51-week treatment period. Study participants who received 90mg and 120mg doses of ION224 achieved the primary endpoint of statistically significant liver histologic improvement. Treatment with the Ionis drug also achieved a key secondary endpoint of MASH resolution without worsening fibrosis.
Viking Therapeutics, Inc. Price

Viking Therapeutics, Inc. price | Viking Therapeutics, Inc. Quote
Zacks Rank
Viking Therapeutics currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Viking Therapeutics, Inc. (VKTX) : Free Stock Analysis Report
Ionis Pharmaceuticals, Inc. (IONS) : Free Stock Analysis Report
Madrigal Pharmaceuticals, Inc. (MDGL) : Free Stock Analysis Report
Akero Therapeutics, Inc. (AKRO) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 