Theravance Pipeline Strong, Dependence on Yupelri a Concern
On Feb 4, we issued an updated research report on Theravance Biopharma, Inc. TBPH. The company is focused on developing treatments targeting various therapeutic areas, such as infectious, respiratory, gastrointestinal, cardiovascular, renal, inflammation and immunology diseases.
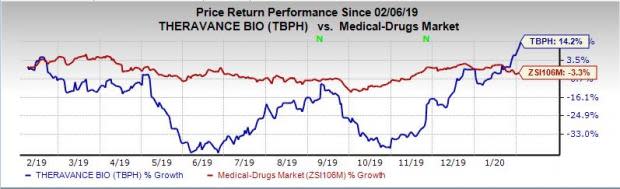
Shares of Theravance have rallied 14.2% in the past year agasint the industry’s decrease of 3.3%.
Theravance received a huge boost when the FDA approved Yupelri, a long-acting muscarinic antagonist (LAMA), as a once-daily, nebulized treatment of chronic obstructive pulmonary disease (COPD) in November 2018.
Theravance and Mylan MYL are collaborating on the development and commercialization of Yupelri. Both companies formally launched Yupelri in early 2019 and the product is witnessing a strong uptake ever since.
The two entities are sharing profits and losses in the United States with respect to the commercialization of Yupelri. While Theravance earns 35% of the profits, Mylan receives the remaining 65%. Notably, the former is entitled to low double-digit royalties on ex-US net sales and the latter recognizes product sales from Yupelri.
Significantly, in November 2018, Theravance completed the sale of its only marketed drug Vibativ to Cumberland Pharmaceuticals.
Theravance holds an economic interest in GlaxoSmithKline's GSK COPD drug Trelegy Ellipta and gains royalties on its sales.
This apart, the company boasts several promising pipeline candidates in its portfolio, which are progressing well.
In December 2019, the company dosed the first patient in a phase II allergen challenge study on its inhaled, lung-selective pan-Janus kinase (JAK) inhibitor TD-8236, which is being developed for treating inflammatory lung diseases.
Theravance has a collaboration agreement with Johnson & Johnson's JNJ subsidiary Janssen to develop its JAK inhibitor TD-1473 for treating inflammatory intestinal diseases. A phase II study on TD-1473 to address Crohn's disease and a phase IIb/III study for ulcerative colitis are ongoing. The company plans to report data from both these studies on TD-1473 by late 2020.
Theravance is also evaluating TD-9855 in a phase III study for treating patients with symptomatic neurogenic orthostatic hypotension (nOH) and a gut-selective irreversible JAK3 inhibitor TD-5202 in a phase I study for addressing inflammatory intestinal diseases.
Successful development and subsequent approval of these candidates will led a huge boost to the company, given the products’ potential to offer transformational value to patients and healthcare providers.
It is important to note that in December 2019, Theravance and Pfizer signed a global license pact for developing Theravance's preclinical program related to skin-targeted, locally-acting pan-Janus kinase inhibitors that can be rapidly metabolized. Per the deal, Theravance will be eligible to receive an upfront payment of $10 million in cash as well as up to an additional $240 million as development and sales-based milestone fees from Pfizer. This can boost growth prospects for Theravance as such collaboration contracts are a consistent source of funds.
However, with the sale of Vibativ, Theravance is now solely dependent on profit-sharing revenues from Mylan for Yupelri and collaboration revenues from J&J for funding its pipeline development. Any agreement termination regarding these in the future might be a massive setback for the company.
Theravance Biopharma, Inc. Price and Consensus
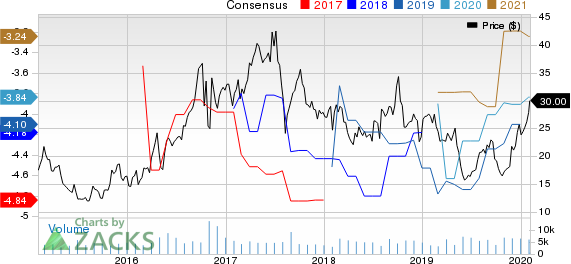
Theravance Biopharma, Inc. price-consensus-chart | Theravance Biopharma, Inc. Quote
Zacks Rank
Theravance currently carries a Zacks Rank #3 (Hold).You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Biggest Tech Breakthrough in a Generation
Be among the early investors in the new type of device that experts say could impact society as much as the discovery of electricity. Current technology will soon be outdated and replaced by these new devices. In the process, it’s expected to create 22 million jobs and generate $12.3 trillion in activity.
A select few stocks could skyrocket the most as rollout accelerates for this new tech. Early investors could see gains similar to buying Microsoft in the 1990s. Zacks’ just-released special report reveals 8 stocks to watch. The report is only available for a limited time.
See 8 breakthrough stocks now>>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
GlaxoSmithKline plc (GSK) : Free Stock Analysis Report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
Theravance Biopharma, Inc. (TBPH) : Free Stock Analysis Report
Mylan N.V. (MYL) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 