Structure Therapeutics (GPCR) Rallying on Obesity Drug Data
Shares of Structure Therapeutics Inc. GPCR surged last week after the company announced positive 12-week top-line data from a phase IIa obesity study as well as top-line data from a capsule-to-tablet PK study on its obesity candidate, GSBR-1290, on Jun 3. Both studies met their primary and secondary endpoints.
GSBR-1290 is a highly selective oral GLP-1 receptor agonist, being evaluated in multiple mid-stage studies for treating healthy overweight or obese individuals.
The capsule-to-tablet PK study was designed to evaluate the tolerability, safety and pharmacokinetics of a new tablet formulation of GSBR-1290 in the given patient population.
Data from the phase IIa obesity study showed that treatment with GSBR-1290 led to a clinically meaningful and statistically significant placebo-adjusted mean decrease in weight of 6.2% at 12 weeks.
Notably, 67% of the patients who were treated with GSBR-1290 experienced a ≥6% reduction in their body weight, while 33% of the patients achieved a ≥ 10% reduction in their body weight compared to none for placebo at 12 weeks of treatment.
Data from the capsule-to-tablet PK study showed that treatment with the tablet formulation of GSBR-1290 led to a placebo-adjusted mean weight loss of up to 6.9% at 12 weeks.
Importantly, treatment with GSBR-1290 was generally safe and well tolerated, with low adverse events related to discontinuation reported. Adverse event-related discontinuations ranged from 5% in the phase IIa obesity study to 11% in the capsule-to-tablet PK study.
Shares of Structure Therapeutics have rallied 54.7% so far this month compared with the industry’s increase of 1.2%.
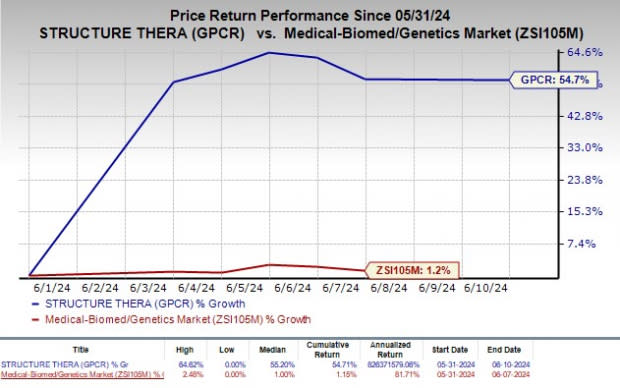
Image Source: Zacks Investment Research
Per Structure Therapeutics, the above data shows that GSBR-1290 can result in substantial weight loss and has the potential to become a best-in-class oral small molecule GLP-1RA in the treatment of obesity.
A phase IIb study of GSBR-1290 in obese patients is expected to be initiated in the fourth quarter of 2024. GPCR has decided to use the tablet formulation of GSBR-1290 for this 36-week global study. The company plans to file an investigational new drug application to the FDA in the third quarter of 2024 to begin the phase IIb obesity study on GSBR-1290.
Obesity has become a global health problem as it can cause heart disease, diabetes and stroke, leading to an exponential increase in demand for obesity medicines.
GLP-1 receptor agonists stimulate insulin secretion and inhibit glucagon release from the pancreas, leading to improved blood sugar control. They also reduce appetite and slow gastric emptying, aiding in weight management.
Some injectable treatments available in the market to treat obesity are Eli Lilly’s LLY Zepbound (tirzepatide) and Novo Nordisk’s NVO Wegovy (semaglutide).
LLY’s Zepbound was launched in November 2023. Despite a short time on the market, Zepbound, a dual GIP and GLP-1 receptor agonist, has become a key top-line driver for Lilly.
Novo Nordisk’s popular GLP-1 receptor agonist, Wegovy, is seeing strong prescription trends and is generating impressive revenues and profits for NVO.
Both Lilly and Novo Nordisk are making efforts to increase the supply capacity for Zepbound and Wegovy in the United States as well as in other international markets.
Amgen AMGN also has a GLP-1 receptor candidate, MariTide (maridebart cafraglutide), for obesity in its pipeline. Last month, AMGN said it was “very encouraged” with the interim data from the phase II study on MariTide. Top-line 52-week data from the phase II study is expected in late 2024.
Amgen is also planning to conduct a comprehensive phase III program on the candidate across obesity, obesity-related conditions and diabetes.
Viking Therapeutics is also progressing with the development of its GIP/GLP-1 agonist called VK2735 for obesity. VK2735 has shown impressive weight loss reductions in studies for both the subcutaneous (phase II) and novel oral (phase I) formulations. Viking Therapeutics plans to advance both formulations into further development later this year.
Structure Therapeutics Inc. Sponsored ADR Price
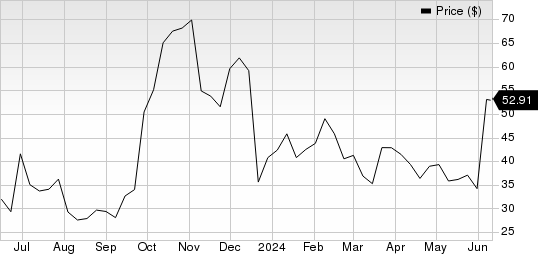
Structure Therapeutics Inc. Sponsored ADR price | Structure Therapeutics Inc. Sponsored ADR Quote
Zacks Rank
Structure Therapeutics currently has a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Novo Nordisk A/S (NVO) : Free Stock Analysis Report
Eli Lilly and Company (LLY) : Free Stock Analysis Report
Amgen Inc. (AMGN) : Free Stock Analysis Report
Structure Therapeutics Inc. Sponsored ADR (GPCR) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 