Single-Dose Radiation Therapy At Time Of Lumpectomy Helps Breast Cancer Patients Overcome Treatment Challenges During Pandemic
TARGIT-IORT long-term data demonstrates equivalent outcomes to traditional 3- to 6-week radiotherapy, significantly reducing COVID-19 risk to patients amidst coming surge
Targeted Intraoperative Radiotherapy (TARGIT-IORT) treatment for breast cancer
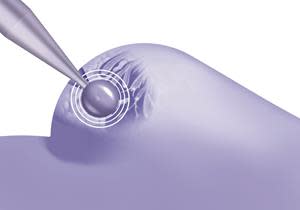
TARGIT-IORT is a single dose of targeted radiation delivered from inside the breast during surgery immediately following the removal of the tumor while the patient remains asleep.
SACRAMENTO, Calif., Dec. 02, 2020 (GLOBE NEWSWIRE) -- The TARGIT Collaborative Group announced today that according to recent studies, an increase in the COVID-19 positivity rate correlates to an increase in mortality from non-coronavirus-related diseases. This includes breast cancer, and researchers believe fear of contracting the virus compels patients to stay home instead of seeking treatment or completing their many week course of post-lumpectomy radiotherapy.
However, surgical and radiation oncologists at the TARGIT Collaborative Group (TCG), a national organization of experts in intraoperative radiotherapy working collaboratively to improve cancer patient care, are countering this unsettling trend with a procedure called Targeted Intraoperative Radiotherapy, or TARGIT-IORT. Unlike external beam radiation therapy (EBRT) which requires up to 30 visits to the radiotherapist, TARGIT-IORT is a single dose of targeted radiation delivered from inside the breast during surgery immediately following the removal of the tumor while the patient remains asleep. For 8 out of 10 patients having TARGIT-IORT during lumpectomy, no further radiotherapy is needed, reducing the COVID-19 risks due to additional outside trips to the hospital during the pandemic.
“As of March, patients have been reluctant to expose themselves to the coronavirus and consequently, they have been less likely to meet their radiation treatment requirements following lumpectomy,” said Dr. Valery Uhl, Radiation Oncologist and President of the TCG. “TARGIT-IORT may negate the challenge of post-surgical compliance issues by reducing or eliminating the patient’s need for external radiation therapy, and this can only positively affect outcomes and recurrence rates.”
Since the beginning of the COVID-19 pandemic, overall deaths in the United States have increased by 20 percent compared to last year, according to a study published in October in the Journal of the American Medical Association (JAMA).1 Crucially, fully one third of these “excess deaths” are due to causes other than COVID-19, leading researchers to conclude that the coronavirus is stopping Americans from seeking and completing medical care for other diseases. Another JAMA study in August found that during the pandemic, the number of patients receiving breast cancer treatment fell 52% in the USA.2
“If elevated coronavirus levels in the spring and summer caused patients to forgo their radiotherapy regimens during those spikes as the JAMA articles indicate, we have every reason to expect compliance challenges to once again increase drastically during the anticipated ‘second wave’ this fall and winter,” Dr. Uhl continued. “Use of the TARGIT-IORT system can counteract that.”
EBRT vs. TARGIT-IORT
External beam radiation therapy (EBRT), sometimes referred to as whole breast radiation therapy, involves treating the entire breast from the outside. Although the radiation therapy is directed to the breast rather than the surrounding tissues, the proximity of the heart, lungs and skin limit the dose of radiation that can be given at any one time. This leads to a prolonged treatment course of three to six weeks following surgery. The TARGIT-IORT treatment, administers the radiation dose from inside the breast at the time of surgery, precisely where it is needed. This allows the radiation oncologist to deliver a much higher dose at one time, avoiding unnecessary radiation to vital adjacent organs such as the lungs and heart. TARGIT-IORT was originally developed at University College London by Professors Jayant S. Vaidya, Michael Baum and Jeffrey S. Tobias and has been well-tested in worldwide clinical trials for more than 20 years.
TARGIT-A TRIAL
The effectiveness of TARGIT-IORT was investigated in an international study called the TARGIT-A Trial, in which the Intrabeam® System from ZEISS was used. This was a randomized clinical trial that compared risk-adapted partial breast single dose targeted intraoperative radiotherapy (TARGIT-IORT) to three to six weeks of post-operative whole breast radiotherapy. 2,298 patients from 32 centers in the UK, USA, Europe, Canada and Australia participated, undergoing lumpectomy for early stage invasive ductal breast cancer. At long term follow-up (median 8.6 years, maximum 18.9 years), the clinical trial demonstrated that there was no difference in the long-term survival without local recurrence; survival without a mastectomy; and survival without distant metastatic disease. Survival from breast cancer was also similar among those who were allocated risk-adapted targeted IORT during lumpectomy compared to those allocated multiple weeks of whole breast radiation after lumpectomy. There were 41% fewer deaths from other causes (such as cardiovascular causes and other cancers). The paper was published in August in BMJ.3
“In the TARGIT-A Trial, risk-adapted TARGIT-IORT was compared head-to-head with whole breast radiotherapy (EBRT), and long-term data found no difference in local and distant breast cancer control, breast preservation or breast cancer mortality,” said Dr. Jayant Vaidya, Professor of Surgery and Oncology at the University College London (UK) and Chief Principal Investigator of the TARGIT-A Trial. “With TARGIT-IORT, there were substantially fewer deaths from other causes such as cardiovascular or lung diseases, and from other cancers. With TARGIT-IORT, most patients complete their breast cancer treatment – lumpectomy surgery and radiotherapy – all at the same time, with lower toxicity. Other benefits include less pain, fewer side effects, cosmetically superior outcome and a better quality of life.”
To learn more about this treatment or to find a TARGIT-IORT provider, please visit the new consumer education website www.targetbreastcancer.org.
ABOUT TARGIT COLLABORATIVE GROUP
The TARGIT Collaborative Group (TCG) is a national organization of radiation oncologists, cancer surgeons, physicists and other experts in intraoperative radiotherapy working collaboratively to improve cancer patient care through education, patient advocacy, mentorship and collaborative research.
REFERENCES
“Excess Deaths from COVID-19 and Other Causes, March-July 2020,” JAMA: The Journal of the American Medical Association, October 12, 2020.
https://jamanetwork.com/journals/jama/fullarticle/2771761“Changes in the Number of US Patients with Newly Identified Cancer Before and During the Coronavirus Disease 2019 (COVID-19) Pandemic” JAMA Network Open, August 4, 2020. https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2768946
“Long term survival and local control outcomes from single dose targeted intraoperative radiotherapy during lumpectomy (TARGIT-IORT) for early breast cancer: TARGIT-A randomized clinical trial”, BMJ, August 19, 2020
https://www.bmj.com/content/370/bmj.m2836.full.pdf
Media Contact:
Drew Avril
drew@coactivepr.com
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/f0395e04-1e49-40a9-8fee-fb20587da5ec

 Yahoo Finance
Yahoo Finance 