Sanofi's (SNY) Dupixent Gets EU Nod for Eczema in Young Kids
Sanofi SNY and partner Regeneron REGN announced that the European Commission has approved their blockbuster medicine, Dupixent, for children six months to five years of age with severe atopic dermatitis, also called eczema. These children are candidates for systemic therapy.
With the approval, Dupixent has become the first and only targeted medicine approved to treat eczema in kids as young as six months old in Europe. The approval is based on data from a phase III study which showed that approximately seven times as many patients in this age group treated with Dupixent experienced clear or almost clear skin and reduced overall disease severity compared to placebo. These patients also achieved rapid itch reduction as early as three weeks after starting therapy.
The approval was expected as, in January, the Committee for Medicinal Products for Human Use (“CHMP”) of the European Medicines Agency had rendered a positive opinion recommending the approval of Dupixent for treating severe atopic dermatitis in infants and young kids. Dupixent was approved by the FDA for atopic dermatitis in children in this age group in June 2022.
In Europe, approximately 80,000 infants and young children have uncontrolled severe atopic dermatitis, which signifies the drug’s significant unmet need for kids under 5 years.
Sanofi stock declined 5.7% in the past year against the industry’s rise of 0.1%.
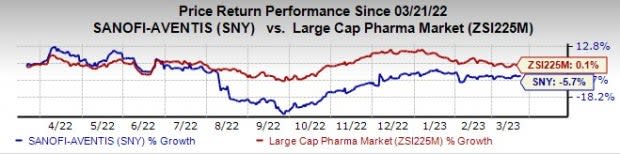
Image Source: Zacks Investment Research
Dupixent is being jointly marketed by Regeneron and Sanofi under a global collaboration agreement. Sanofi records global net product sales of Dupixent, while Regeneron records its share of profits/losses in connection with the global sales of the drug.
Sanofi and Regeneron’s Dupixent is now approved in the United States and EU for five type II inflammatory diseases, namely severe chronic rhinosinusitis with nasal polyposis, severe asthma, moderate-to-severe atopic dermatitis, eosinophilic esophagitis and prurigo nodularis. The frequent label expansion approvals are driving the drug’s sales higher.
Dupixent has become the key driver of the top line for Sanofi. Dupixent generated global product sales of $8.3 billion in 2022, which were recorded by Sanofi, representing growth of 43.8% at a constant exchange rate. With outside U.S. revenues accelerating and multiple approvals for new indications and expansion in younger patient populations expected, its sales are likely to be higher.
Dupixent is now annualizing at close to €9.0 billion in sales after around five years on the market. Sanofi expects Dupixent to achieve more than €13 billion in peak sales and €10 billion in 2023.
Sanofi and Regeneron are also studying dupilumab in late-stage studies in a broad range of diseases driven by type II inflammation, like bullous pemphigoid, chronic spontaneous urticaria, and chronic obstructive pulmonary disease.
Sanofi currently has a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Stocks to Consider
Some better-ranked drugmakers/biotech companies are Novo Nordisk NVO and Ligand Pharmaceuticals LGND, both with a Zacks Rank of 1.
Estimates for Novo Nordisk’s 2023 earnings per share have increased from $4.15 to $4.43. Estimates for 2024 have jumped from $4.38 per share to $5.19 in the past 60 days. The stock has surged 30.3% in the past year.
Novo Nordisk beat earnings expectations in three of the trailing four quarters. The company delivered a four-quarter earnings surprise of 3.00%, on average.
Estimates for Ligand Pharmaceuticals’ 2023 earnings per share have increased from $3.30 to $4.15 over the past 60 days while that for 2024 have jumped from $3.10 per share to $4.28 over the same timeframe. The stock has declined 36.4% in the past year.
Ligand Pharmaceuticals beat earnings expectations in one of the trailing four quarters. The company delivered a four-quarter negative earnings surprise of 10.07%, on average.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
Sanofi (SNY) : Free Stock Analysis Report
Novo Nordisk A/S (NVO) : Free Stock Analysis Report
Ligand Pharmaceuticals Incorporated (LGND) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 