Regeneron's (REGN) Antibody Cocktail for COVID-19 Approved in Japan
Regeneron Pharmaceuticals, Inc. REGN announced that its antibody cocktail, casirivimab and imdevimab, for the treatment of COVID-19 has been approved in Japan. The cocktail, known as REGEN-COV in the United States, was granted full approval in Japan under the name Ronapreve.
The antibody cocktail was granted a Special Approval Pathway under article 14-3 of the Pharmaceuticals and Medical Devices Act in Japan.
The approval was based on positive results from a phase III study in high-risk non-hospitalized patients, which showed that the antibody cocktail reduced the risk of hospitalization or death by 70% along with results from a phase I study that examined the safety, tolerability and pharmacokinetics in Japanese people.
We note that Regeneron has collaborated with Roche RHHBY to increase the global supply of the antibody cocktail. Per the agreement, Roche is primarily responsible for the development and distribution of the product outside the United States.
Last year, Chugai obtained the development and exclusive commercialization rights in Japan from Roche. Chugai is working with the Japanese government to ensure an appropriate and timely supply of the antibody cocktail.
The FDA has granted an Emergency Use Authorization (EUA) to REGEN-COV to treat mild-to-moderate COVID-19 in adults and pediatric patients (12 years of age and older weighing ≥40 g) with positive results of direct SARS-CoV-2 viral testing, and those at high risk of progression to severe COVID-19, including hospitalization or death.
The FDA recently updated the EUA for REGEN-COV, lowering the dose to 1,200 mg (600 mg casirivimab and 600 mg imdevimab), which is half the dose originally authorized.
Regeneron is in discussions with the FDA to expand the current EUA to other populations, including prevention and hospitalized patient settings.
The company's shares have gained 21.2% in the year so far against the industry’s decline of 3%.
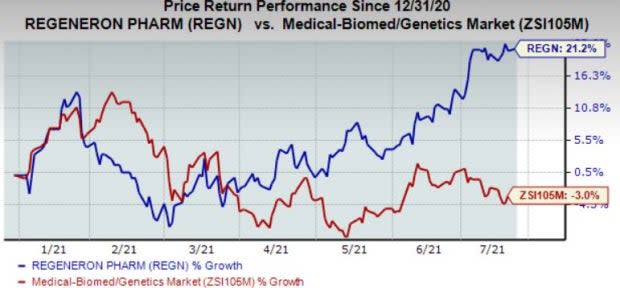
Image Source: Zacks Investment Research
Regeneron has announced results from multiple late-stage studies demonstrating the ability of REGEN-COV to reduce the burden of COVID-19, from prevention to hospitalization.
Despite the increasing rates of vaccination, the pandemic continues to wreak havoc, giving rise to the need for treatments of those infected particularly against the variants of concern.
The FDA granted an EUA to GlaxoSmithKline plc GSK and Vir Biotechnology, Inc. VIR ’s sotrovimab, an investigational single-dose monoclonal antibody, for the treatment of mild-to-moderate COVID-19 in adults and pediatric patients (12 years of age and older weighing at least 40 kg).
Regeneron currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
GlaxoSmithKline plc (GSK) : Free Stock Analysis Report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Vir Biotechnology, Inc. (VIR) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 