Pharma Stock Roundup: JNJ, NVS Q2 Earnings, PFE, ABBV, LLY & MRK FDA Updates
This week, J&J JNJ started the pharma sector earnings season with strong second-quarter results. Novartis NVS announced mixed second-quarter financial results. The FDA announced delays in decisions on regulatory filings of JAK inhibitor drug candidates of AbbVie ABBV, Pfizer PFE and Lilly LLY for atopic dermatitis or eczema indication amid increased scrutiny of JAK inhibitor class of drugs.
Recap of the Week’s Most Important Stories
J&J & Novartis Begin Pharma Q2 Earnings Season: J&J reported stronger-than-expected second-quarter earnings, beating estimates on both counts, driven by strong sales and earnings growth across all three segments. Its Pharmaceuticals unit continued to perform above market levels. The recovery in the Medical Devices unit continued while sales rebounded in its Consumer Health unit. J&J raised its previously issued guidance range for earnings and sales for 2021 to include $2.5 billion contribution from its COVID-19 vaccine as well as expected growth in the base business.
Novartis reported mixed results for the second quarter as earnings topped expectations while sales missed the same. Sales in Innovative Medicines rose 10% at constant currency driven by the strong performance of drugs like Entresto, Cosentyx and Zolgensma. Sales in the Sandoz division rose 5% as the business started to stabilize. The company still expects net sales in 2021 to grow in low- to mid-single digits.
Roche’s RHHBY sales rose 14% year over year in the second quarter and 8% in the first half of 2021. Sales in the Pharmaceuticals unit declined 3% in the first half hurt by biosimilar competition for legacy drugs like MabThera/Rituxan, Herceptin and Avastin and the impact of the pandemic. However, the Diagnostics division sales rose 51% in the first half of 2021 due to a surge in COVID-19 diagnostics tests, which peaked in the second quarter. In 2021, Roche continues to expect sales to grow in the low- to mid-single digit range in 2021 at constant exchange rates.
FDA Delays Decision on Lilly, Pfizer & AbbVie’s Eczema Treatments: Lilly and partner Incyte announced that the FDA will not be able to meet the PDUFA date on their supplemental new drug application (sNDA) seeking approval of Olumiant (baricitinib) for moderate-to-severe atopic dermatitis (AD). The FDA was expected to give its decision in the third quarter of 2021. Olumiant is already approved in Europe and Japan for atopic dermatitis. Olumiant is presently approved to treat rheumatoid arthritis in several countries.
AbbVie also announced that the FDA did not give its decision on the sNDA seeking approval of its JAK inhibitor drug, Rinvoq (upadacitinib) for moderate-to-severe AD on the PDUFA action date. Rinvoq is presently approved for treating moderate-to-severe rheumatoid arthritis.
The FDA also said it will not be able to meet the PDUFA date for the NDA of Pfizer’s JAK inhibitor candidate, abrocitinib for moderate-to-severe AD. The FDA was expected to give its decision on the NDA in the third quarter of 2021. The FDA also delayed the decision on Pfizer’s sNDA seeking approval of its marketed JAK inhibitor, Xeljanz for active ankylosing spondylitis (AS).
This is the second time that the FDA has delayed its decision on expanded use of arthritis drugs, Olumiant and Rinvoq, for the AD indication as well as on Pfizer’s NDA for abrocitinib for AD and Xeljanz’s sNDA for AS.
The FDA is currently reviewing Pfizer’s post-marketing study, ORAL Surveillance, on Xeljanz (tofacitinib), in patients with rheumatoid arthritis, which it cited as the reason for all the above delays. We remind investors that in February, the FDA issued a statement that Xeljanz may increase the risk of heart-related problems and cancer. Last month, the FDA similarly informed AbbVie that it will not give its decision on sNDAs on Rinvoq for active psoriatic arthritis and active ankylosing spondylitis in adults, citing the same reason. The extension of review periods for sNDAs and other negative updates for JAK inhibitor drugs have raised concerns regarding safety issues of this class of drugs.
FDA Approves Merck’s 15-Valent Pneumococcal Vaccine: The FDA granted approval to Merck’s investigational 15-valent pneumococcal conjugate vaccine for active immunization for the prevention of invasive pneumococcal disease caused by 15 serotypes in adults 18 years of age and older. The vaccine will be marketed by the trade name of Vaxneuvance. Vaxneuvance includes pneumococcal serotypes, 22F and 33F, which are not included in the currently licensed 13-valent conjugate vaccines. An application seeking approval of the vaccine is under review in the EU.
The FDA also approved a combination of Merck’s blockbuster cancer drug, Keytruda and partner Eisai’s Lenvima for certain types of advanced endometrial carcinoma based on data from the pivotal phase III KEYNOTE-775 study. The approval is for treating endometrial carcinoma that is not microsatellite instability-high or mismatch repair deficient in patients who have disease progression following prior systemic therapy and are not candidates for curative surgery or radiation.
The European Commission granted approval to Merck and Bayer’s soluble guanylate cyclase (sGC) stimulator, Verquvo/vericiguat, for treating symptomatic chronic heart failure in patients with reduced ejection fraction (HFrEF). The drug was approved by the FDA in January this year.
Glaxo’s Data from Studies on Daprodustat: Glaxo GSK announced positive headline data from five studies of the phase III ASCEND programme of daprodustat in patients with anemia due to chronic kidney disease. The data showed that daprodustat met its primary efficacy endpoint in each study, showing an improvement in haemoglobin (Hgb) levels in untreated patients. The candidate was also successful in maintaining Hgb levels in patients treated with an erythropoietin stimulating agent (ESA), a standard treatment in such patient population. Data from key cardiovascular outcomes studies for non-dialysis (ASCEND-ND) and dialysis patients (ASCEND-D) showed that daprodustat was non-inferior to an ESA in the risk of Major Adverse Cardiovascular Events (MACE), the co-primary endpoint of both studies. Daprodustat is currently approved in Japan as Duvroq for patients with renal anemia.
Pfizer’s COVID Vaccine Manufacturing Partner for Africa: Pfizer announced a collaboration with South African biotech, Biovac to manufacture and distribute its COVID vaccine among more than 50 African countries. Biovac will source the drug substance from facilities in Europe. The production of finished doses will commence in 2022 with a goal of making more than 100 million finished doses annually.
Pfizer and its partner Valneva have completed recruitment in a phase II study for their jointly developed vaccine candidate, VLA15, for Lyme disease, an infectious disease caused by the Borrelia bacterium, which is spread by ticks. The phase II study (VLA15-221) on VLA15 will include 620 healthy participants, both adult and pediatric subjects (5-17 year). The study will evaluate VLA15 at two different schedules (Month 0-2-6 or Month 0-6) receiving the selected dose of 180µg. Top-line data from the study will be announced in the first half of 2022
Pfizer announced the collaboration with Valneva to co-develop and commercialize VLA15 in April 2020. It has already announced positive top-line results from two phase II studies in over 800 healthy adults.
FDA’s Breakthrough Tag to Roche/AbbVie’s Venclexta for MDS: The FDA granted Breakthrough Therapy status to AbbVie/Roche’s blockbuster cancer drug Venclexta in combination with azacitidine for the treatment of patients with myelodysplastic syndromes (MDS), a rare group of blood cancers. The prestigious tag was bestowed based on interim data from the phase Ib M15-531 study investigating Venclexta/Venclyxto plus azacitidine for previously untreated higher-risk MDS.
FDA Approves Sanofi’s Sleeping Sickness Drug: Sanofi announced that the FDA has granted approval for fexinidazole, an oral treatment for human African trypanosomiasis (HAT) or sleeping sickness in patients 6 years of age and older and weighing at least 20 kg. The 10-day once-a-day treatment has proven to be efficacious for both states of the disease.
The FDA also granted Fast Track status to Bayer’s pluripotent stem cell-derived dopaminergic neuron therapy, DA01 for advanced Parkinson’s disease (PD). The candidate is being evaluated in a phase I study.
The NYSE ARCA Pharmaceutical Index rose 0.9% in the last five trading sessions.

Large Cap Pharmaceuticals Industry 5YR % Return
Large Cap Pharmaceuticals Industry 5YR % Return
Here’s how the eight major stocks performed in the last five trading sessions.
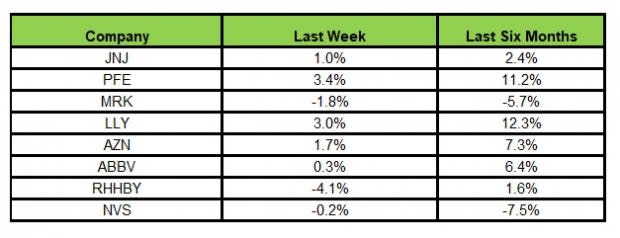
Image Source: Zacks Investment Research
In the last five trading sessions, Pfizer rose the most (3.4%) while Roche recorded the maximum decline (4.1%).
In the past six months, Lilly has recorded the maximum gain (12.3%) while Novartis declined the most (7.5%)
(See the last pharma stock roundup here: NVO & LLY’s Small Acquisitions, JNJ’s Sunscreens Recall)
What's Next in the Pharma World?
Watch out for Merck, Pfizer and others’ Q2 earnings and regular pipeline and regulatory updates next week.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
GlaxoSmithKline plc (GSK) : Free Stock Analysis Report
Novartis AG (NVS) : Free Stock Analysis Report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Eli Lilly and Company (LLY) : Free Stock Analysis Report
AbbVie Inc. (ABBV) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 