Novartis (NVS) Announces Positive Data on Oncology Drug
Novartis AG NVS recently announced results from the late-stage RATIONALE 306 study on tislelizumab.
Novartis is developing tislelizumab in collaboration with BeiGene BGNE. Novartis has the right to develop, manufacture and commercialize tislelizumab in North America, Europe and Japan.
The multi-regional phase III, randomized, placebo-controlled, double-blind study, RATIONALE 306, is evaluating tislelizumab in combination with chemotherapy versus chemotherapy in patients with unresectable, locally advanced recurrent or metastatic esophageal squamous cell carcinoma (ESCC). Approximately 649 study participants were randomized 1:1 to receive either tislelizumab plus chemotherapy or chemotherapy plus placebo.
Data from the study showed tislelizumab plus chemotherapy significantly enhanced overall survival (OS) as a first-line treatment for adult patients with unresectable, locally advanced or metastatic ESCC, regardless of PD-L1 status.
The combination demonstrated a median OS of 17.2 months compared with 10.6 months in patients receiving chemotherapy plus placebo and reduced the risk of death by 34%.
In patients with PD-L1 score ≥10% (secondary endpoint), tislelizumab plus chemotherapy showed a median OS of 16.6 months versus 10.0 months in patients receiving chemotherapy plus placebo and reduced the risk of death by 38%.
We note that tislelizumab is a uniquely designed anti-PD-1 monoclonal antibody which is being evaluated in pivotal clinical trials across a broad array of solid tumors. It is currently under review in the United States and Europe for advanced or metastatic ESCC after prior chemotherapy. The European Medicines Agency (EMA) is also reviewing tislelizumab for advanced or metastatic non-small cell lung cancer (NSCLC) after prior chemotherapy and in combination with chemotherapy for previously untreated advanced or metastatic NSCLC.
Novartis has also signed an option, collaboration and license agreement with BeiGene for ociperlimab (BGB-A1217).
Shares of Novartis have lost 3.3% so far this year against the industry’s growth of 4.9%.
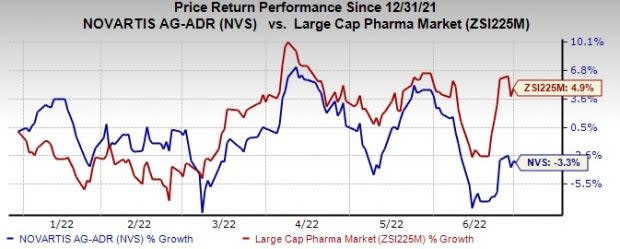
Image Source: Zacks Investment Research
Approval of new drugs bodes well for Novartis. The FDA earlier approved Novartis’ targeted radioligand therapy, Pluvicto, for treating progressive, PSMA-positive metastatic castration-resistant prostate cancer. Vijoice (alpelisib), used for treating patients aged two years and above with severe manifestations of PIK3CA-Related Overgrowth Spectrum (PROS), requiring systemic therapy, was also approved by the regulatory body.
Novartis has a strong and diverse portfolio. Solid momentum in key brands like psoriasis drug Cosentyx, cardiovascular drug Entresto, gene therapy Zolgensma, the oncology portfolio and the launch of Kesimpta continue to boost performance.
Novartis streamlined its business significantly and is looking forward to focusing on its core business. NVS is still evaluating whether to retain or sell its generic business and expects to provide an update on the same by the end of this year.
Per Bloomberg, Novartis is keen on a spinoff of its generic arm Sandoz over a potential sale to private equity firms as a challenging environment for leveraged buyouts makes a potential sale to private equity more difficult. Moreover, Novartis is also keen on a separate listing of the $25 billion Sandoz business.
Zacks Rank & Stocks to Consider
Novartis currently carries a Zacks Rank #3 (Hold).
A couple of better-ranked stocks are Sesen Bio SESN and Geron Corporation GERN. While Sesen sports a Zacks Rank #1 (Strong Buy), Geron carries a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank stocks here.
Loss estimates for SESN for 2022 have narrowed to 44 cents from a loss of 46 cents in the past 60 days. Sesen surpassed estimates in all of the trailing four quarters, the average surprise being 69.94%.
Loss estimates for GERN for 2022 have narrowed by 6 cents in the past 60 days. Geron surpassed estimates in three of the trailing four quarters, the average surprise being 1.07%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Novartis AG (NVS) : Free Stock Analysis Report
Geron Corporation (GERN) : Free Stock Analysis Report
BeiGene, Ltd. (BGNE) : Free Stock Analysis Report
SESEN BIO, INC. (SESN) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 