Merit Medical (MMSI) Recalls Syringes Made by Jiangsu Shenli
Merit Medical Systems, Inc. MMSI recently issued an urgent medical device recall notice related to specific products that contain plastic syringes manufactured by a Chinese manufacturer, Jiangsu Shenli Medical Production Co Ltd. (Jiangsu Shenli).
After discovering that Jiangsu Shenli was selling syringes that were not the same as the approved device, the FDA earlier this year issued an import alert and warning letter against the manufacturer.
More on the News
The FDA has released a Field Safety Communication about syringes made by Jiangsu Shenli in China. Merit Medical’s supplier notified the company that they had supplied them with syringes from Jiangsu Shenli, who had been issued a warning letter stating that all but one size/type of their syringes were not adequately authorized for sale by the FDA.
The FDA also mentioned "growing evidence of potential harm" for Jiangsu Shenli syringes in the warning letter. FDA recommends in its Field Safety Communication that continued use of these syringes is “absolutely necessary” until alternatives are available to “closely monitor for leaks, breakage, and other problems.” Leaks or breakage may result in a delay in treatment.
The FDA stumbled upon Jiangsu Shenli when looking into the possibility of China-made plastic syringes leaking, shattering, or having other quality issues. The officials accused Jiangsu Shenli of marketing 29 different sizes and configurations of piston syringes without clearance or approval.
Regarding the distribution of Jiangsu Shenli syringes, Merit Medical recently handed out an urgent medical device recall notice. The company claimed that the syringes were part of several different products. Customers are requested to cease using and sharing the syringes with immediate effect, but they can keep utilizing the other components of the kits.
Actions Taken by Merit Medical
Merit Medical is taking action to shift from Jiangsu Shenli syringes immediately. In the interim, to avoid product shortages, the company is likely to ship products containing the Jiangsu Shenli syringes until the transition has been completed and those products will already have the recall label applied. Products that customers continue to receive without the recall label and not identified on the Customer Response Form will not include Jiangsu Shenli syringes.
Per Merit Medical, adverse reactions or quality problems experienced with the use of products may be reported to the FDA’s MedWatch Adverse Event Reporting program either online, by regular mail, or by fax.
Notable Developments
In May, the company announced the U.S. commercial release of the basixSKY Inflation Device. The device is available as a standalone solution and in kits with Merit Medical’s Angioplasty Packs, configured to offer complementing AccessPLUS, Honor, and PhD hemostasis valves. The basixSKY is the latest addition to Merit Medical’s comprehensive inflation device portfolio, which includes both digital and analog devices.
Merit Medical also received the FDA’s 510(k) clearance for its Siege Vascular Plug. The company also announced the launch of its Bearing nsPVA Express Prefilled Syringe in the United States and Australia.
In February, the company announced the FDA approval for the SCOUT MD Surgical Guidance System. With the ability to target tumor sites in various dimensions for accurate excision and successful surgeries, the new guiding system represents a substantial development in the treatment of breast cancer. SCOUT MD adds to Merit Medical’s oncology portfolio.
Price Performance
For the past six months, MMSI’s shares have gained 13.6% compared with the industry’s rise of 3.2%. The S&P 500 increased 15.1% in the same time frame.
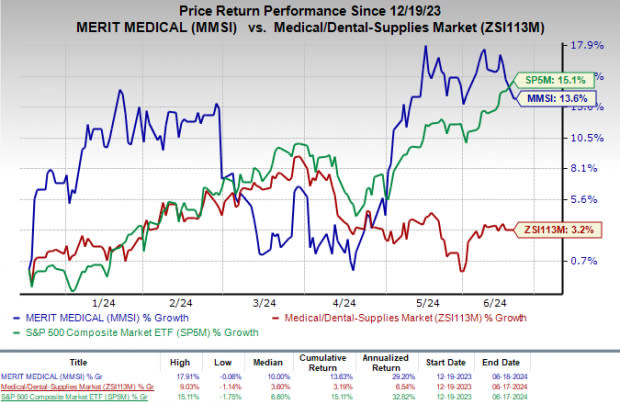
Image Source: Zacks Investment Research
Zacks Rank & Stocks to Consider
MMSI carries a Zacks Rank #3 (Hold) at present.
Some better-ranked stocks in the broader medical space that have announced quarterly results are DaVita DVA, Ecolab ECL and Boston Scientific Corporation BSX.
DaVita, carrying a Zacks Rank #2 (Buy) at present, has an estimated long-term growth rate of 13.6%. DVA’s earnings surpassed estimates in each of the trailing four quarters, with the average surprise being 29.4%. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
DaVita’s shares have gained 44% compared with the industry’s 20.4% rise in the past year.
Ecolab, carrying a Zacks Rank of 2 at present, has an estimated long-term growth rate of 13.3%. ECL’s earnings surpassed estimates in each of the trailing four quarters, the average surprise being 1.7%.
Ecolab’s shares have rallied 33.8% against the industry’s 9.3% decline in the past year.
Boston Scientific reported first-quarter 2024 adjusted earnings per share of 56 cents, which beat the Zacks Consensus Estimate by 9.8%. Revenues of $3.86 billion surpassed the Zacks Consensus Estimate by 4.9%. It currently carries a Zacks Rank #2.
Boston Scientific has a long-term estimated growth rate of 12.5%. BSX’s earnings surpassed estimates in the trailing four quarters, the average surprise being 7.5%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Boston Scientific Corporation (BSX) : Free Stock Analysis Report
Ecolab Inc. (ECL) : Free Stock Analysis Report
DaVita Inc. (DVA) : Free Stock Analysis Report
Merit Medical Systems, Inc. (MMSI) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance