Medtronic's (MDT) EV ICD Pivotal Study Shows Favorable Outcome (Revised)
Medtronic MDT recently declared the findings of its investigational Extravascular Implantable Cardioverter–Defibrillator (EV ICD) Study. These were presented as late-breaking science at Heart Rhythm 2023 in New Orleans. The results confirm that the system provides the advantages of a device with lead outside the heart and continues to provide pause prevention pacing and anti-tachycardia pacing to avoid shocks with prolonged follow-ups.
By providing a contemporary view of the real-world impact of ICDs, the EV ICD study underscores the importance of indicated patients receiving these potentially life-saving devices.
About the EV ICD System
The Medtronic EV ICD system is an implantable defibrillator, which is designed to treat dangerously fast heart rhythms that can lead to sudden cardiac arrests. This is the first system that offers anti-tachycardia pacing, pause prevention pacing, and a device similar in size, shape and battery longevity to transvenous ICDs.
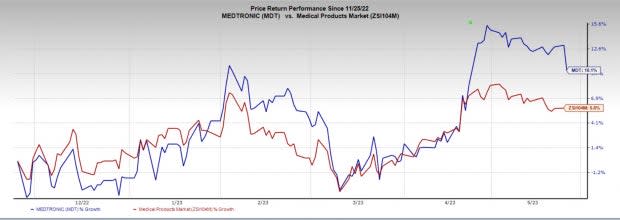
Image Source: Zacks Investment Research
Study Findings
Of the 299 implanted patients followed through an average of 17.1 months, an estimated 6.8% of patients showed to have experienced appropriate therapy by 18 months, with 19 patients experiencing 80 spontaneous, appropriately treated arrhythmic episodes. Of the discrete episodes treated with shock, 100% were successfully terminated.
The rate of freedom from major EV ICD system-or-procedure-related complications through 18 months was 91.9%. The most common major complication was lead dislodgment, identified within 120 days post-implant, mostly related to the lead-anchoring technique. Inappropriate shocks occurred in 35 patients throughout all follow-ups.
Industry Prospects
Per a Research report, the global implantable cardioverter defibrillator market size was valued at $3.3 billion and is expected to witness a CAGR of 5.3% up to 2030.
Recent Developments
In May 2023, Medtronic received FDA approval for its Micra AV2 and Micra VR2 — the next generation of its industry-leading miniaturized, leadless pacemakers. The world's smallest pacemakers provide longer battery life and easier programming than prior Micra pacemakers, while delivering the expanded benefits of leadless pacing compared to traditional pacemakers.
In April 2023, Medtronic announced the FDA approval of its MiniMed 780G system with the Guardian 4 sensor, which requires no fingerstick while in SmartGuard technology. This milestone marks the approval of the only system with meal detection technology that provides automatic adjustments and corrections to sugar levels every five minutes.
Price Performance
In the past six months, MDT shares have increased 10.1% compared with the industry’s rise of 5.8%.
Zacks Rank and Key Picks
Medtronic currently carries a Zacks Rank #3 (Hold).
Some better-ranked stocks in the overall healthcare sector are Penumbra PEN, Lantheus LNTH and Neuronetics STIM. While Penumbra and Lantheus each sport a Zacks Rank #1 (Strong Buy), Neuronetics carries a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank stocks here.
Penumbra’s stock has risen 118.3% in the past year. The Zacks Consensus Estimate for Penumbra’s earnings per share (EPS) has increased from $1.47 to $1.56 for 2023 and from $2.51 to $2.56 for 2024 in the past seven days.
PEN’s earnings beat estimates in each of the trailing four quarters, the average surprise being 109.42%. In the last reported quarter, the company registered an earnings surprise of 109.09%.
The Zacks Consensus Estimate for Lantheus’ 2023 EPS has increased from $4.95 to $5.60 in the past 30 days. Shares of the company have improved 51% in the past year against the industry’s 31.2% decline.
LNTH’s earnings beat estimates in each of the trailing four quarters, the average surprise being 25.77%. In the last reported quarter, the company recorded an earnings surprise of 13.95%.
Estimates for Neuronetics’ loss per share have narrowed from $1.32 to $1.29 for 2023 in the past seven days. Shares of the company have risen 14.8% in the past year compared with the industry’s 1.8% growth.
STIM’s earnings beat estimates in each of the trailing four quarters, the average surprise being 19.61%. In the last reported quarter, Neuronetics delivered an earnings surprise of 2.56%.
(We are reissuing this article to correct a mistake. The original article, issued on May 24, 2023, should no longer be relied upon.)
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Medtronic PLC (MDT) : Free Stock Analysis Report
Penumbra, Inc. (PEN) : Free Stock Analysis Report
Lantheus Holdings, Inc. (LNTH) : Free Stock Analysis Report
Neuronetics, Inc. (STIM) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 