Masimo's (MASI) New FDA Approval to Boost Patient Monitoring
Masimo Corporation MASI received the FDA’s 510(k) clearance for its patient-worn, continuous multi-parameter vital signs monitor, Radius VSM. The monitor, designed on a modular platform, enables clinicians to track a wide variety of physiological measurements, including Masimo SET pulse oximetry, non-invasive blood pressure, temperature, respiration rate and electrocardiography.
The Radius VSM is currently available in Europe. Following the latest FDA approval, U.S. hospitals will now gain access to the same.
The latest regulatory approval is a major stepping stone for Masimo’s real-time patient monitoring business and is likely to solidify its position in the niche space on a global scale.
Significance of the Approval
Radius VSM is expected to combine the reliability and accuracy of larger bedside monitors with the comfort and freedom of a wearable device. This will likely allow ambulation and movement while ensuring patients remain continuously monitored. Radius VSM’s flexibility and expandability are expected to enable easy scalability to match each patient’s unique monitoring needs and level of acuity across the continuum of care and to accommodate surges in patient volume.
Radius VSM can connect wirelessly to Masimo bedside monitors like Root and to the Masimo Hospital Automation platform, simplifying clinical workflows by automating patient data transfer to remote monitoring systems like Masimo Patient SafetyNet and electronic medical records. This will likely enable its use as part of a patient surveillance system and ensure up-to-date physiological data is available to clinicians throughout the hospital.
Per an expert familiar with the use of Radius VSM, it is a solution that combines the advantages of a comprehensive monitoring platform with the autonomy of telemetry. This provides the patient with the safety and freedom to make their hospital stay more humanized.
Per Masimo’s management, Radius VSM includes features like unique scalability, advanced connectivity and a broad range of accurate and automated continuous measurements, among others, in a wearable device that can be quickly and easily deployed anywhere in the hospital. This is expected to make it an innovative tool for clinicians everywhere.
Industry Prospects
Per a report by Allied Market Research, the global patient monitoring devices market was estimated to be $25,768.56 million in 2019 and is anticipated to reach $44,861.56 million by 2027 at a CAGR of 4.4%. Factors like technological advancements and a growing preference for telehealth services are expected to drive the market.
Given the market potential, the latest FDA approval is likely to provide a significant boost to Masimo’s business globally.
Notable Developments
Last month, Masimo announced the global expansion of the HEOS platform, which enables an always-on connection to the Masimo Health secure cloud for 4 million devices, thus empowering consumers with an enhanced health-tracking experience.
The same month, Masimo reported its first-quarter 2023 results, wherein it registered a robust uptick in the top line and its healthcare business. The company also recorded robust order shipments during the reported quarter. The expansion of the company’s installed base was also witnessed.
Also, in May, Masimo announced that Cambridge University Hospitals NHS Foundation Trust, U.K. adopted the Masimo W1 advanced health tracking watch for use in its telehealth and telemedicine programs.
Price Performance
Shares of Masimo have gained 18% in the past year compared with the industry’s 2.3% rise and the S&P 500's 4.5% growth.
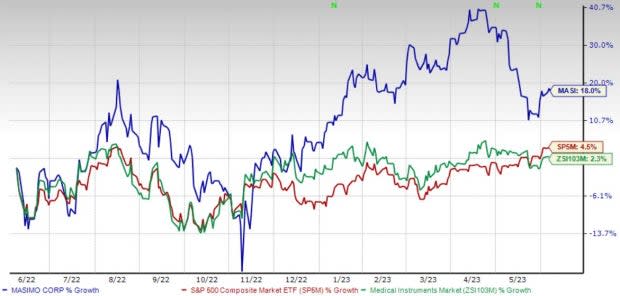
Image Source: Zacks Investment Research
Zacks Rank & Other Key Picks
Currently, Masimo carries a Zacks Rank #2 (Buy).
A few other top-ranked stocks in the broader medical space are Hologic, Inc. HOLX, Merit Medical Systems, Inc. MMSI and Boston Scientific Corporation BSX.
Hologic, carrying a Zacks Rank #2 at present, has an estimated growth rate of 5.1% for fiscal 2024. HOLX’s earnings surpassed estimates in all the trailing four quarters, the average being 27.3%. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Hologic has gained 4.7% compared with the industry’s 2.3% rise in the past year.
Merit Medical, carrying a Zacks Rank #2 at present, has an estimated long-term growth rate of 11%. MMSI’s earnings surpassed estimates in all the trailing four quarters, the average surprise being 20.2%.
Merit Medical has gained 43.3% compared with the industry’s 8.2% rise over the past year.
Boston Scientific, carrying a Zacks Rank #2 at present, has an estimated long-term growth rate of 11.5%. BSX’s earnings surpassed estimates in two of the trailing four quarters and missed in the other two, the average surprise being 1.9%.
Boston Scientific has gained 29.3% against the industry’s 31.3% decline over the past year.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Boston Scientific Corporation (BSX) : Free Stock Analysis Report
Hologic, Inc. (HOLX) : Free Stock Analysis Report
Masimo Corporation (MASI) : Free Stock Analysis Report
Merit Medical Systems, Inc. (MMSI) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 