IceCure Medical (ICCM) Submits Final Cryoablation Data to FDA
IceCure Medical ICCM recently announced that the company had submitted final data to the FDA requesting market authorization for its ProSense System.
ProSense is designed to provide cryoablation, an outpatient technique that treats breast cancer in 20–40 minutes. It requests FDA approval to use cryoablation and adjuvant hormone therapy in the treatment of individuals with early-stage T1 invasive breast cancer.
Price Performance
In the past six months, ICCM shares have gained 41.8% compared with the industry’s rise of 9.7%. The S&P 500 has gained 17.3% in the same time frame.
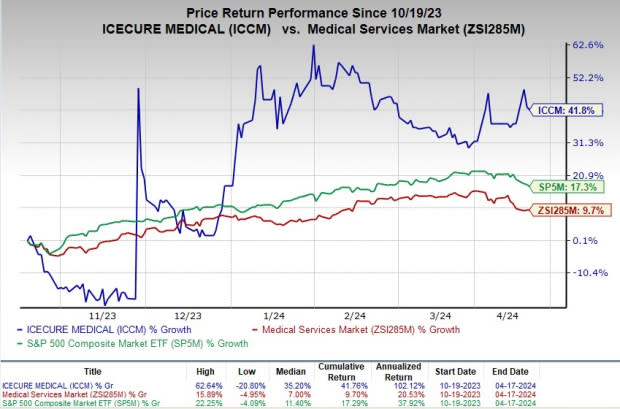
Image Source: Zacks Investment Research
More on the ProSense System
The ProSense Cryoablation System is intended to provide a minimally invasive treatment option to destroy tumors by freezing them. For optimal effectiveness in destroying tumors in benign and malignant lesions, such as those of the breast, kidney, lung, and liver, the method innovatively uses liquid nitrogen to form sizable lethal zones.
By decreasing discomfort, surgical risks, and consequences, ProSense improves value for both patients and providers. The system makes office-based breast tumor procedures quickly and easily accessible with its lightweight, transportable design and use of liquid nitrogen.
ProSense already has clearance in the United States. The system has clearance for other indications, which include treating benign tumors of the breast and tumors in the kidney and liver.
More on the Data Submitted
In 2022, the FDA rejected IceCure's request for de novo categorization of ProSense, which is used to treat individuals with low-risk, early-stage breast cancer. In January 2024, the FDA consented to reopen the de novo file. The FDA responded positively, offering IceCure a possible route to clearance.
Together with a sub-analysis, the company submitted a five-year follow-up dataset from the ICE3 research. The FDA also received data from real-world sources, such as independent third-party study results and post-market commercial use.
The clinical trial for cryoablation of low-risk, early-stage malignant breast cancers using liquid nitrogen was carried out as part of the ICE3 research. This innovative study evaluated IceCure's minimally invasive 20-to-40-minute outpatient cryoablation procedure; the five-year recurrence-free rates were in line with expectations and showed similar outcomes to lumpectomy, the current standard of care for patients with early-stage breast cancer.
Based on the data, 96.3% of the patients in the subgroup treated with ProSense cryoablation and hormone therapy were thought to be free of local recurrence at the five-year follow-up evaluation. Comparing this ICE3 study results to patients treated with lumpectomy followed by hormone therapy in the LUMINA study, which reported a 97.7% recurrence-free rate at five-year follow-up, and the PRISMA meta-study, which included Lumina, reported a 97.19% recurrence-free rate at five-year follow-up reveals similar outcomes in five-year recurrence rates.
Industry Prospects
Per a report by Global Market Insights, the global cryoablation devices market size was valued at more than $690 million in 2022 and is expected to reach more than $1.5 billion by 2032 at a growth rate of 8.5%.
Demand for cryoablation devices is expected to be driven by factors such as patients' and surgeons' growing preference for minimally invasive treatment procedures because of their benefits, which include faster recovery times, less downtime, and increased patient comfort.
IceCure Medical Ltd. Price
IceCure Medical Ltd. price | IceCure Medical Ltd. Quote
Zacks Rank & Stocks to Consider
ICCM carries a Zacks Rank #3 (Hold) at present.
Some better-ranked stocks in the broader medical space are DaVita Inc. DVA, Cardinal Health, Inc. CAH and Cencora, Inc. COR.
DaVita, sporting a Zacks Rank #1 (Strong Buy), has an estimated long-term growth rate of 12.1%. DVA’s earnings surpassed estimates in each of the trailing four quarters, with the average surprise being 35.6%. You can see the complete list of today’s Zacks #1 Rank stocks here.
DaVita’s shares have gained 58.3% compared with the industry’s 18.9% rise in the past year.
Cardinal Health, flaunting a Zacks Rank of 1 at present, has an estimated long-term growth rate of 14.2%. CAH’s earnings surpassed estimates in each of the trailing four quarters, with the average being 15.6%.
Cardinal Health has gained 51.9% compared with the industry’s 3.2% rise in the past year.
Cencora, carrying a Zacks Rank of 2 (Buy) at present, has an estimated long-term growth rate of 9.8%. COR’s earnings surpassed estimates in each of the trailing four quarters, with the average surprise being 6.7%.
Cencora’s shares have rallied 51.5% compared with the industry’s 3.6% rise in the past year.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
DaVita Inc. (DVA) : Free Stock Analysis Report
Cardinal Health, Inc. (CAH) : Free Stock Analysis Report
Cencora, Inc. (COR) : Free Stock Analysis Report
IceCure Medical Ltd. (ICCM) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 