Here's What's Driving AbbVie's (ABBV) Outperformance This Year
AbbVie ABBV stock has risen 17.9% this year so far compared with an increase of 8.5% for the industry.
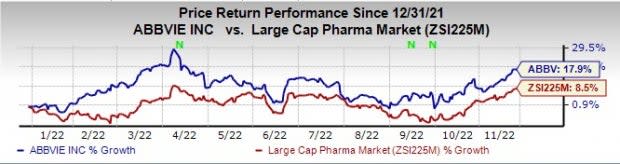
Image Source: Zacks Investment Research
AbbVie has become one of the top-most pharma companies after it acquired Botox maker Allergan in a cash-and-stock deal for $63 billion in May 2020. The deal has transformed AbbVie’s portfolio by lowering its dependence on Humira, its flagship product, which has already lost patent protection in Europe and is due to face biosimilar competition in the United States in 2023. AbbVie has one of the most popular cancer drugs in its portfolio, Imbruvica, which it markets in partnership with J&J JNJ. Its newest immunology drugs Skyrizi (risankizumab) and Rinvoq (upadacitinib) position it well for long-term growth.
Humira continues to witness strong demand trends in the United States despite new mechanisms of action and competition from indirect biosimilars. Currently approved for several indications, Humira sales have increased consistently, backed by robust demand trends.
Among AbbVie’s newer medicines, immunology drugs, Skyrizi and Rinvoq have established outstanding launch trajectories bolstered by their approval in new indications. The drugs generated sales of $3.59 billion and $1.75 billion in the first nine months of 2022, rising 75.6% and 54.5%, respectively, year over year. Both Skyrizi and Rinvoq were approved for active psoriatic arthritis in 2021/early 2022. Rinvoq was approved for atopic dermatitis, ulcerative colitis, ankylosing spondylitis and non-radiographic axial SpA and Skyrizi for Crohn’s disease in 2022. With approvals for such new indications, sales of these drugs could be higher in 2023/2024 and have the potential to replace Humira when generics are launched in the United States in 2023. AbbVie expects the combined sales of Skyrizi and Rinvoq to be more than $15 billion by 2025.
Meanwhile, new migraine drugs, Ubrelvy and Qulipta, represent a $1 billion-plus peak sales opportunity each.
In its oncology franchise, the strong growth of Venclexta sales is making up for lower U.S. sales of J&J-partnered Imbruvica. U.S. sales of AbbVie/J&J’s Imbruvica are being hurt by lower new patient starts in CLL due to delayed recovery from the pandemic and increasing competition from newer therapies.
AbbVie markets Venclexta/Venclyxto in partnership with Roche RHHBY. AbbVie and Roche jointly commercialize Venclexta in the United States while AbbVie markets it outside the country.
Label expansion approvals in the past couple of years have expanded the eligible patient population of AbbVie/Roche’s Venclexta/Venclyxto significantly, which is boosting sales from the drug. AbbVie expects to file a regulatory application seeking approval of Venclexta in relapsed/refractory multiple myeloma in 2023 and in myelodysplastic syndrome or MDS in 2024.
Besides, AbbVie has an exciting and diverse pipeline of promising new therapies in both blood cancers and solid tumors like navitoclax for myelofibrosis, epcoritamab, a CD3xCD20 bispecific, across several B-cell malignancies, including diffuse B cell and follicular lymphomas; ABBV-383, a BCMA CD3 bispecific, being studied for multiple myeloma and Teliso-V, a promising c-Met ADC being studied for nonsquamous non-small cell lung cancer. AbbVie and partner Genmab’s GMAB regulatory application seeing accelerated approval of epcoritamab in patients with relapsed/refractory large B-cell lymphoma is under priority review in the United States and under review in the EU. Regulatory decisions for AbbVie/Genmab’s epcoritamab are expected in 2023.
AbbVie also has an impressive late-stage pipeline with several early/mid-stage candidates that have blockbuster potential. Several data readouts are expected in 2023, which could be catalysts for the stock.
AbbVie has its share of problems. Increasing existing and new competition, the persistent weakness of the CLL market and potential erosion from recent unfavorable changes to the NCCN guideline preference for Imbruvica in CLL are expected to hurt Imbruvica’s performance in 2023.
Economic pressure is impacting consumers' discretionary spending, which is slowing down treatment procedures and hurting sales in the aesthetics portfolio, mainly Juvederm and body contouring portfolio products.
There are concerns about AbbVie’s long-term sales growth once Humira generics enter the U.S. market in 2023.
Nonetheless, strong sales of Venclexta, Skyrizi and Rinvoq, regular pipeline success, including further label expansions of Skyrizi and Rinvoq and successful collaboration deals should keep the stock afloat in 2023.
Zacks Rank
AbbVie currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
AbbVie Inc. (ABBV) : Free Stock Analysis Report
Genmab AS Sponsored ADR (GMAB) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 