Gamida (GMDA) Up on Omidubicel BLA Rolling Submission Plan
Gamida Cell Ltd. GMDA announced that the FDA has agreed to the initiation of rolling submission of a biologics license application (“BLA”) for its lead pipeline candidate, omidubicel. The BLA will seek approval for omidubicel as a potential life-saving allogeneic hematopoietic stem cell (bone marrow) transplant solution for patients with blood cancers. The company will start the filing of the BLA in the first half of 2022.
The company has successfully completed a phase III study that evaluated omidubicel in patients with hematologic malignancies undergoing a bone marrow transplant compared to patients who received a standard umbilical cord blood transplant. Following the completion of the late-stage study, the company had a type B pre-BLA meeting with the FDA for the discussion of the regulatory procedure for gaining approval for the candidate.
Gamida completed the pre-BLA meeting with the FDA in November last year. Although the regulatory body did not request additional clinical data during the meeting, it requested the company to submit a revised analysis of the manufacturing data generated at Gamida Cell’s wholly-owned commercial manufacturing facility to evaluate the comparability of the commercial-grade product with the candidate used in the phase III study.
In the recent update earlier this week, Gamida confirmed that it reached an alignment with the FDA that the commercial-grade product and clinical-study product are analytically comparable. Following the establishment of the analytical comparability, the FDA agreed to the rolling submission of the BLA for omidubicel.
Shares of Gamida surged 22.5% on Jan 19, following the promising regulatory progress. However, Gamida’s shares have declined 69.6% in the past year compared with the industry’s decrease of 36.8%.
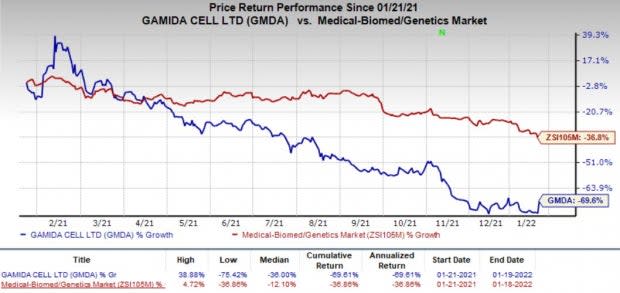
Image Source: Zacks Investment Research
Gamida had plans to initiate the rolling submission of the BLA in the fourth quarter of 2020, followed by its launch in the second half of 2021. However, regulatory hurdles delayed the process, hurting its stock price.
Following a potential FDA approval, omidubicel is likely to become the first advanced cell therapy for allogeneic bone marrow transplant available for blood cancer patients in need of stem cell transplant.
Data from the phase III study evaluating omidubicel demonstrated that treatment with the candidate resulted in a significantly shorter duration of hospitalization, intensive care unit stays, consultant visits, procedures, and transfusions. The candidate enjoys Breakthrough Therapy and Orphan Drug designation as bone marrow transplant graft in the United States, suggesting a significant unmet need in the targeted indication.
Gamida also has a second cell therapy candidate, GDA-201, in its pipeline, which is being evaluated to treat patients with non-Hodgkin lymphoma and multiple myeloma. However, the FDA has placed a clinical hold on a proposed phase I/II study on the candidate citing concerns on donor eligibility procedures and sterility assay qualification. The company is engaged with the FDA for the removal of the clinical hold and initiation of the study as early as possible.
Gamida Cell Ltd. Price

Gamida Cell Ltd. price | Gamida Cell Ltd. Quote
Zacks Rank and Stocks to Consider
Gamida currently carries a Zacks Rank #3 (Hold). Some better-ranked stocks in the biotech sector are Regeneron Pharmaceuticals REGN, Repligen Corporation RGEN and Adaptive Biotechnologies ADPT, all carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Estimates for Regeneron have improved from earnings per share of $46.98 to $47.77 for 2022 over the past 60 days.
Regeneron delivered an earnings surprise of 28.93%, on average, in the last four quarters. Shares of REGN have gained 14% in the past year.
Estimates for Repligen’s 2022 earnings have been revised upward from $3.20 to $3.23 over the past 60 days.
Repligen delivered an earnings surprise of 49.21%, on average, in the last four quarters. Shares of RGEN have gained 2.1% in the past year.
Estimates for Adaptive Biotechnologies have narrowed from a loss of $1.53 to $1.51 for 2022 over the past 60 days.
Adaptive Biotechnologies delivered an earnings surprise of 11.21%, on average, in the last four quarters.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
Repligen Corporation (RGEN) : Free Stock Analysis Report
Adaptive Biotechnologies Corporation (ADPT) : Free Stock Analysis Report
Gamida Cell Ltd. (GMDA) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 