FATE's Q4 Loss Narrower Than Expected, Pipeline in Focus
Fate Therapeutics FATE reported a loss of 58 cents per share in the fourth quarter of 2022, narrower than the Zacks Consensus Estimate of a loss of 86 cents and the year-ago loss of 72 cents.
The loss narrowed year-over-year due to higher revenues.
The company earned collaboration revenues of $44 million in the fourth quarter, which easily surpassed the Zacks Consensus Estimate of $20 million and were up from $17 million reported in the year-ago quarter. Revenues are primarily derived from Fate’s collaborations with Janssen, a unit of Johnson & Johnson JNJ and Ono Pharmaceutical.
R&D expenses surged to $87.2 million from $69.5 million in the year-ago quarter.
G&A expenses jumped to $21.6 million from $16.9 million in the year-ago quarter.
Cash, cash equivalents and investments at the end of the fourth quarter were $441.2 million.
Shares of Fate have plunged 82.2% in the past year compared with the industry’s decline of 11%.
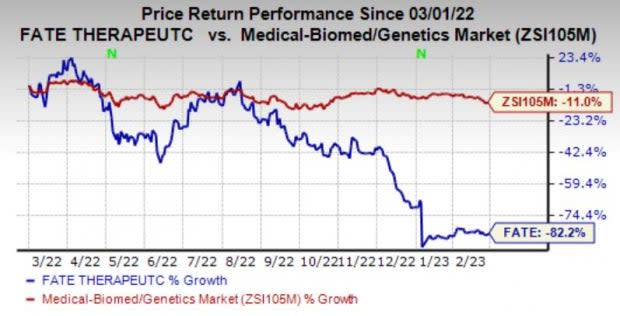
Image Source: Zacks Investment Research
Pipeline Update
FATE is focused on the development and manufacture of universal, off-the-shelf cell products using its proprietary induced pluripotent stem cell (iPSC) product platform. Its immuno-oncology pipeline includes off-the-shelf, iPSC-derived natural killer (NK) cells and T-cell product candidates. These candidates are designed to synergize with well-established cancer therapies, including immune checkpoint inhibitors and monoclonal antibodies and to target tumor-associated antigens using chimeric antigen receptors (CARs).
In December 2022, FATE presented interim phase I data from the first nine patients treated with a single dose of FT576 — its multiplexed-engineered, BCMA-targeted CAR NK cell product candidate for relapsed or refractory multiple myeloma. Data from the single-dose treatment cohorts in heavily pre-treated patients showed encouraging clinical evidence of BCMA-targeted activity and a favorable safety profile, indicating the potential for administration in the outpatient setting.
Fate is currently enrolling two-dose treatment cohorts as monotherapy and in combination with CD38-targeted mAb therapy at 300 million cells per dose and, upon clearance, the company plans to open and assess three-dose treatment cohorts starting at 1 billion cells per dose.
Fate intends to submit an investigational new drug (IND) application to the FDA in mid-2023 to commence a phase I study of FT522 in combination with CD20-targeted mAb therapy for the treatment of B-cell lymphoma.
Under its collaboration with ONO Pharmaceutical, Fate is co-developing FT825/ONO-8250, a multiplexed-engineered, iPSC-derived CAR T-cell product candidate targeting human epidermal growth factor receptor 2 (HER2)-expressing solid tumors. The parties are conducting IND-enabling activities for FT825/ONO-8250 and expect to submit an IND application to the FDA in 2023 to commence a phase I study for the treatment of patients with HER2-positive solid tumors.
Fate Therapeutics, Inc. Price, Consensus and EPS Surprise
Fate Therapeutics, Inc. price-consensus-eps-surprise-chart | Fate Therapeutics, Inc. Quote
Other Key Updates
In January 2023, Fate announced the termination of its agreement with JNJ's Janssen. Both companies entered into an agreement in April 2020 to collaborate on the development of iPSC-derived CAR NK- and CAR T-cell product candidates for the treatment of cancer. During the fourth quarter of 2022, Janssen exercised its second commercial option for a collaboration product under the agreement, for which Fate expects to receive a $10 million milestone payment. Additionally, Janssen authorized the submission of and the FDA allowed an IND application for a first collaboration product for the treatment of B-cell lymphoma during the fourth quarter. FATE expects to receive a $3 million milestone payment for the same.
As a result of the collaboration’s termination, during the first quarter of 2023, Fate and Janssen are winding down all collaboration activities, including discontinuing the development of all collaboration products, at the expense of Janssen.
Fate has completed a strategic review of its NK cell programs and elected to advance its most innovative and differentiated product candidates. As a result of this program prioritization as well as the termination of the Janssen collaboration, Fate is discontinuing the development of its FT516, FT596, FT538 and FT536 NK cell programs and is reducing its workforce in the first quarter of 2023 to approximately 220 employees. Fate will most likely incur charges of approximately $12 million to $16 million for severance and other employee termination-related costs for the same.
Fate currently carries a Zacks Rank #2 (Buy). Some other top-ranked stocks in the sector are Amarin AMRN and Geron GERN, both carrying the same rank as Fate. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
AMRN’s loss estimates for 2022 have been flat but have narrowed by 2 cents for 2023 in the past 60 days. Amarin surpassed earnings in two of the trailing four quarters and missed in the remaining two, with the average negative earnings surprise being 14.29%.
GERN’s loss estimates for 2022 have been flat too but have narrowed by 2 cents for 2023 in the past 60 days. Geron surpassed earnings in two of the trailing four quarters and missed in the remaining two, with the average earnings surprise being 1.07%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Johnson & Johnson (JNJ) : Free Stock Analysis Report
Geron Corporation (GERN) : Free Stock Analysis Report
Amarin Corporation PLC (AMRN) : Free Stock Analysis Report
Fate Therapeutics, Inc. (FATE) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 