Eli Lilly (LLY) to Ramp Up Indiana Site for Mounjaro, Zepbound
Eli Lilly and Company LLY announced that it is more than doubling its investment in its manufacturing site in Lebanon, IN, from $3.7 billion to $9 billion. This increased investment of $5.3 billion will allow it to increase its capacity to manufacture active pharmaceutical ingredients (API) for its popular tirzepatide drugs, Mounjaro and Zepbound.
The increased investment is expected to create 200 new jobs for highly skilled workers at the Lebanon site and 5,000 construction jobs during the site's development. Lilly plans to begin manufacturing drugs at the Lebanon site toward the end of 2026, with production expected to increase through 2028.
Lilly’s Mounjaro was launched in mid-2022 for type II diabetes. Zepbound was launched in November 2023 for patients who were obese or overweight with weight-related comorbidities. Despite a short time on the market, Mounjaro and Zepbound have become key top-line drivers for Lilly, with demand for weight loss drugs rising rapidly. Mounjaro and Zepbound generated $1.81 billion and $517.4 billion, respectively, in the first quarter of 2024.
Mounjaro and Zepbound include the same compound tirzepatide, a dual GIP and GLP-1 receptor agonist (GIP/GLP-1 RA). The GLP-1 segment is a very important class of drugs for multiple cardiometabolic diseases and is gaining significant popularity. GLP-1 drugs work by mimicking the hormone GLP-1, resulting in weight loss, lowering hemoglobin A1c (HbA1c) and reducing cardiovascular risks.
Lilly’s stock has risen 38.5% year to date compared with an increase of 15.9% for the industry.
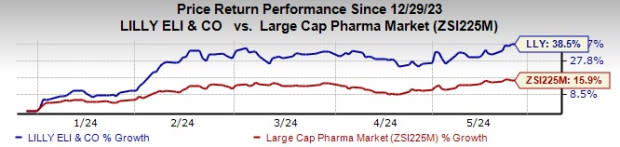
Image Source: Zacks Investment Research
Lilly is seeing huge demand for Mounjaro and Zepbound, which is causing a supply shortage. Demand is exceeding supply despite the company's increasing production volumes. The supply constraints are affecting Lilly’s ability to launch Mounjaro and Zepbound in new markets. Lilly expects the supply to be tight in the near to mid-term as demand exceeds supply.
Lilly is investing in new advanced manufacturing plants and lines in the United States and Europe to increase supply. Since 2020, Lilly has committed more than $18 billion to building new manufacturing sites as well as updating existing manufacturing facilities.
Lilly expects meaningful increases in shipment volumes of Mounjaro and Zepbound from the second half of 2024. On the first-quarter conference call held last month, Lilly said it expects the production of sellable doses of incretin products in the second half of 2024 to be at least 1.5 times the sellable doses in the second half of 2023.
Greater visibility and confidence in its production expansion plans and strong demand for Mounjaro and Zepbound pushed Lilly to raise its sales and earnings guidance ranges for 2024 despite mixed first-quarter performance.
Obesity has become a global health problem as it can cause other diseases like heart disease, diabetes and stroke, leading to an exponential increase in demand for obesity medicines.
Lilly’s tirzepatide medicines face strong competition from Novo Nordisk’s NVO semaglutide. Semaglutide is approved as Ozempic pre-filled pen and Rybelsus oral tablet for type II diabetes and as Wegovy injection for weight management. Despite supply challenges, Wegovy is seeing strong prescription trends and is generating impressive revenues and profits. Novo Nordisk is also making efforts to increase the supply capacity for Wegovy in the United States as well as other international markets.
Viking Therapeutics VKTX is also progressing with the development of its GIP/GLP-1 agonist called VK2735 for obesity. VK2735 has shown impressive weight loss reductions in studies for both the subcutaneous (phase II) and novel oral (phase I) formulations. Viking Therapeutics plans to advance both formulations into further development later this year.
Amgen AMGN also has a GLP-1 receptor candidate, MariTide (maridebart cafraglutide), for obesity in its pipeline. Earlier this month, Amgen said it was “very encouraged” with the interim data from the phase II study on MariTide. Top-line 52-week data from the phase II study is expected in late 2024. Amgen is planning to conduct a comprehensive phase III program on the candidate across obesity, obesity-related conditions and diabetes
Lilly currently has a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Eli Lilly and Company Price and Consensus
Eli Lilly and Company price-consensus-chart | Eli Lilly and Company Quote
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Novo Nordisk A/S (NVO) : Free Stock Analysis Report
Eli Lilly and Company (LLY) : Free Stock Analysis Report
Amgen Inc. (AMGN) : Free Stock Analysis Report
Viking Therapeutics, Inc. (VKTX) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance