Dr. Reddy's (RDY) Meets Goals in Actemra Biosimilar Study
Dr. Reddy’s Laboratories RDY announced that the phase I study for its biosimilar candidate of Roche’s RHHBY drug Actemra (tocilizumab), DRL_TC, met its primary and secondary endpoints. The phase I study evaluated the pharmacokinetic equivalence, safety and immunogenicity of an intravenous (“IV”) formulation of DRL_TC in comparison with the reference drug.
Tocilizumab is developed and marketed by Roche in the United States as Actemra and in the EU as RoActemra, for the treatment of rheumatoid arthritis. In the phase I study for DRL_TC, both Actemra and RoActemra were sourced as reference medicinal products from the United States and EU, respectively. The phase I study comprised three arms in which, either DRL_TC, Actemra or RoActemra, were administered by the IV Route to normal healthy male volunteers.
So far this year, shares of Dr. Reddy’s have climbed 9% compared with the industry’s 3.3% rise.
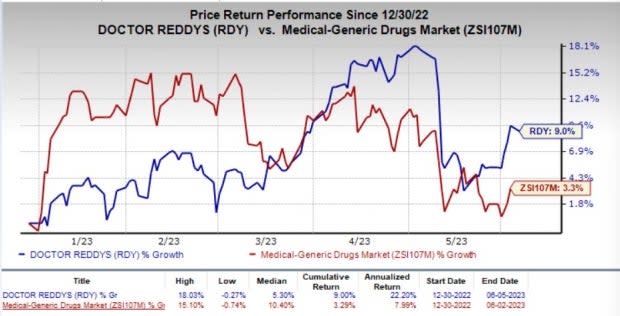
Image Source: Zacks Investment Research
The phase I study successfully demonstrated the pharmacokinetic equivalence of DRL_TC to Actemra and RoActemra. The study also confirmed the similarity between Dr. Reddy’s biosimilar candidate and Roche’s originally marketed products in terms of pharmacodynamic parameters. Additionally, no noteworthy differences in safety and immunogenicity profiles were observed across the treatment with DRL_TC, Actemra or RoActemra in the three treatment groups.
Dr. Reddy’s is developing DRL_TC as both intravenous and subcutaneous formulations. In December 2022, the company reported meeting primary and secondary endpoints in its phase I study evaluating the same parameters of a subcutaneous formulation of DRL_TC, in comparison with Actemra and RoActemra.
The company is now gearing up to initiate a global phase III study to compare the efficacy, safety, tolerability and immunogenicity of DRL_TC with the reference product in patients with moderate to severe active rheumatoid arthritis.
Management believes that the development of both subcutaneous and intravenous biosimilar formulations of tocilizumab will fulfill the company’s aim to reach more patients around the world, suffering from rheumatoid arthritis and other diseases.
In January 2023, Dr. Reddy’s reported the successful completion of the full set of clinical studies of DRL_RI, its biosimilar candidate for Roche’s Rituxan, to support approval plea in the United States, Europe and other regions. The company is developing DRL_RI for various indications, including the treatment of adult patients with rheumatoid arthritis, non-Hodgkin's lymphoma, chronic lymphocytic leukemia, pemphigus vulgaris, granulomatosis with polyangiitis and microscopic polyangiitis.
Currently, Dr. Reddy’s is preparing to file a biologics license application with the FDA for approval in the United States as well as respective regulatory filing in the EU, for DRL_RI.
Dr. Reddy's Laboratories Ltd Price and Consensus
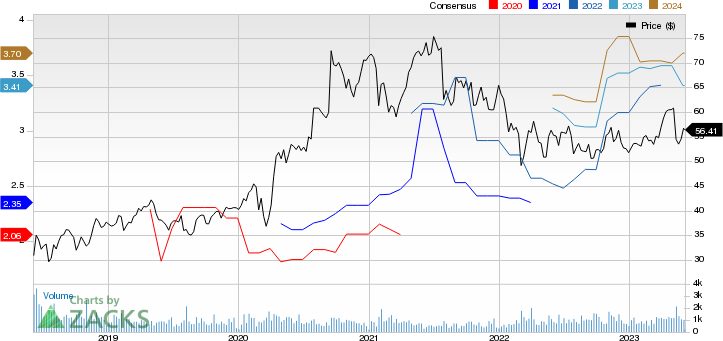
Dr. Reddy's Laboratories Ltd price-consensus-chart | Dr. Reddy's Laboratories Ltd Quote
Zacks Rank and Stocks to Consider
Dr. Reddy’s currently has a Zacks Rank #3 (Hold).
A couple of better-ranked stocks in the biotech sector are Adaptimmune Therapeutics ADAP and Immunogen IMGN, both carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
In the past 90 days, the Zacks Consensus Estimate for Adaptimmune Therapeutics’ 2023 loss per share has narrowed from 78 cents to 46 cents. During the same period, the estimate for Adaptimmune Therapeutics’ 2024 loss per share has narrowed from $1.10 to 56 cents. In the year so far, shares of Adaptimmune Therapeutics have fallen by 28.7%.
ADAP beat estimates in each of the trailing four quarters, delivering an average earnings surprise of 36.89%.
In the past 90 days, the Zacks Consensus Estimate for ImmunoGen’s 2023 loss per share has narrowed from 83 cents to 55 cents. During the same period, the estimate for ImmunoGen’s 2024 loss per share has narrowed from 58 cents to 31 cents. In the year so far, shares of ImmunoGen have rallied by 209.9%.
IMGN beat estimates in two of the trailing four quarters, missing the mark on the other two occasions, delivering an average earnings surprise of 7.09%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Dr. Reddy's Laboratories Ltd (RDY) : Free Stock Analysis Report
Roche Holding AG (RHHBY) : Free Stock Analysis Report
ImmunoGen, Inc. (IMGN) : Free Stock Analysis Report
Adaptimmune Therapeutics PLC (ADAP) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 