Bristol Myers' (BMY) Opdivo Combo Gets EU Nod for First-Line RCC
Bristol-Myers Squibb Company BMY announced that the European Commission (“EC”) has approved its PD-1 inhibitor Opdivo (nivolumab) in combination with Exelixis’ EXEL Cabometyx (cabozantinib) for first-line treatment of adult patients with advanced renal cell carcinoma (“RCC”).
The EC nod was based on data from the phase III CheckMate -9ER study which evaluated Opdivo + Cabometyx in the given patient population. Data from the same showed that treatment with Opdivo + Cabometyx led to superior efficacy as compared to Pfizer’s PFE Sutent (sunitinib) across three key endpoints – progression-free survival which was the primary endpoint, and objective response rate as well as overall survival.
Also, the combo of Opdivo plus Cabometyx was generally well tolerated, with a low rate of treatment-related discontinuations.
Please note that Opdivo in combination with Cabometyx was approved for first-line treatment of patients with advanced RCC in the United States in January this year.
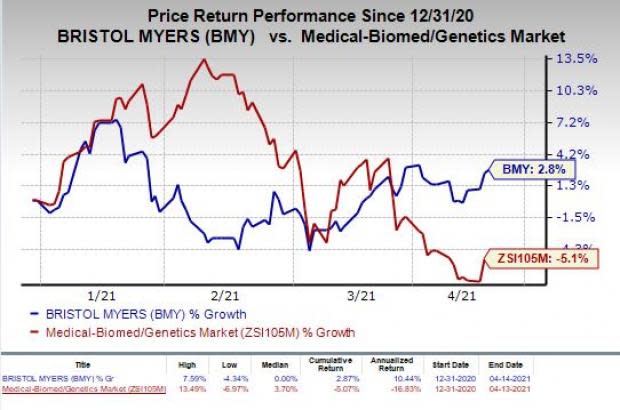
Shares of Bristol Myers have increased 2.8% so far this year against the industry’s decrease of 5.1%.
We remind investors that, apart from advanced RCC, Opdivo is approved in several countries for various other cancer indications.
Opdivo generated sales worth $6.9 billion in 2020, reflecting a decrease of 3% year over year. The drug faces stiff competition from Merck’s MRK Keytruda and Roche’s Tecentriq in key indications.
Please note that Keytruda in combination with Inlyta is also approved for the first-line treatment of patients with advanced RCC. This is likely to be a tough competition for Opdivo in the given market space.
Zacks Rank
Bristol-Myers currently carries a Zacks Rank #3 (Hold). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
More Stock News: This Is Bigger than the iPhone!
It could become the mother of all technological revolutions. Apple sold a mere 1 billion iPhones in 10 years but a new breakthrough is expected to generate more than 77 billion devices by 2025, creating a $1.3 trillion market.
Zacks has just released a Special Report that spotlights this fast-emerging phenomenon and 4 tickers for taking advantage of it. If you don't buy now, you may kick yourself in 2022.
Click here for the 4 trades >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
To read this article on Zacks.com click here.

 Yahoo Finance
Yahoo Finance 