Is a Beat on the Cards for Regeneron's (REGN) Q1 Earnings?
Regeneron Pharmaceuticals, Inc. REGN is scheduled to release first-quarter 2023 results on May 4, before the opening bell.
The company has an impressive track record, beating on earnings in all the past four quarters. In the last reported quarter, it beat earnings expectations by 29.75%. It surpassed earnings estimates by 20.44%, on average, in the trailing four quarters.
Regeneron Pharmaceuticals, Inc. Price, Consensus and EPS Surprise
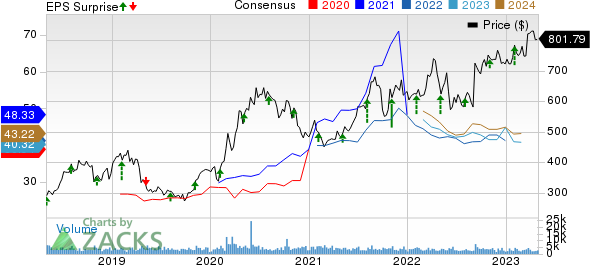
Regeneron Pharmaceuticals, Inc. price-consensus-eps-surprise-chart | Regeneron Pharmaceuticals, Inc. Quote
What Our Model Predicts
Our proven model predicts an earnings beat for Regeneron this time around. The combination of a positive Earnings ESP and a Zacks Rank #1 (Strong Buy), 2 (Buy) or 3 (Hold) increases the odds of an earnings beat.
Earnings ESP: Earnings ESP for REGN is +4.41% as the Zacks Consensus Estimate currently stands at $9.25 per share and the Most Accurate Estimate is currently pegged at $9.66. You can uncover the best stocks to buy or sell before they're reported with our Earnings ESP Filter.
Zacks Rank: The company currently carries a Zacks Rank #3.
Factors at Play
A major chunk of Regeneron’s revenues comes from the sales of its lead drug, Eylea, which is approved for various ophthalmology indications (neovascular age-related macular degeneration, diabetic macular edema and macular edema, among others). Eylea was developed in collaboration with Bayer AG.
Regeneron records net product sales of Eylea in the United States, while Bayer records net product sales of the drug outside the country. Regeneron also records its share of profits/losses in connection with sales of Eylea outside the United States.
Eylea's sales have been strong and it has maintained momentum for the company in the past few quarters. However, sales were negatively impacted in the fourth quarter due to a short-term shift to off-label use of Avastin, a temporary closing in the fourth quarter of 2022 of a not-for-profit fund that provides patient co-pay assistance and an increase in sales-related deductions. Regeneron stated that it has substantially recovered from the issue in the fourth quarter, but Eylea’s sales in the first quarter have most likely faced competitive pressure. The Zacks Consensus Estimate for Eylea’s sales in the United States is pegged at $1.52 billion.
Apart from Eylea, investors will focus on the asthma drug Dupixent’s performance, sales of which are recorded by Sanofi SNY. Regeneron has a collaboration agreement with Sanofi for drugs like Dupixent and Kevzara. While Sanofi records sales, Regeneron records its share of profits/losses in connection with global sales of Dupixent and Kevzara. Profits from Dupixent sales have been REGN’s primary growth driver in the last few quarters.
Sales of Dupixent were up 39.7% in the first quarter, as recorded by Sanofi and hence Regeneron is likely to have recorded incremental profits in the yet-to-be-reported quarter. Dupixent has maintained its stellar performance, driven by continued strong demand in the approved indications, atopic dermatitis (AD), asthma, chronic rhinosinusitis with nasal polyposis (CRSwNP), eosinophilic esophagitis and prurigo nodularis. The label expansion of the drug in the past few months has boosted sales further and is likely to have resulted in incremental revenues for Regeneron in the first quarter.
Investors will focus on the performance of Libtayo and the PCSK9 inhibitor Praluent. Effective Jul 1, 2022, Regeneron records net product sales of Libtayo and will pay Sanofi a royalty on such sales. Increased sales of Libtayo on-label expansions have most likely boosted the top line. The Zacks Consensus Estimate for Libtayo’s sales is pegged at $166 million.
Regeneron records net product sales of Praluent in the United States. Sanofi records net product sales of the drug outside the United States and pays Regeneron a royalty on such sales. Total sales in the previous quarter witnessed a 30% increase. However, it’s mostly unlikely for the first quarter to have recorded such growth.
In January 2022, the FDA revised the authorizations for a few monoclonal antibody treatments, including Regeneron’s REGEN-COV (casirivimab and imdevimab), as data indicated that these treatments are highly unlikely to be active against the Omicron variant. REGEN-COV is a cocktail of two monoclonal antibodies (casirivimab and imdevimab, also known as REGN10933 and REGN10987, respectively). Therefore, REGEN-COV is currently unauthorized for use in U.S. states, territories or jurisdictions. Hence, this revision dented sales in 2022 from this stream and sales are likely to be negligible in the first quarter.
Recent Updates
In March 2023, the FDA approved Evzeeka (evinacumab-dgnb) as an adjunct to other lipid-lowering therapies to treat children aged 5 to 11 with homozygous familial hypercholesterolemia (HoFH). The approval extends Evkeeza to children aged 5 to 11 with homozygous familial hypercholesterolemia (HoFH), an inherited condition characterized by extremely high low-density lipoprotein cholesterol (LDL-C).
The European Commission (EC) approved Libtayo (cemiplimab) in combination with platinum-based chemotherapy for first-line treatment of adult patients with advanced non-small cell lung cancer (NSCLC) with ≥1% PD-L1 expression.
Share Price Performance
Regeneron’s shares have gained 11.1% in the year so far against the industry’s decline of 6.2%.
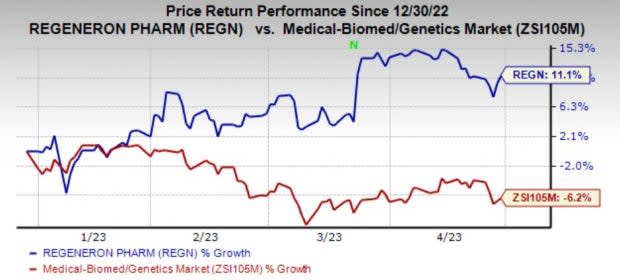
Image Source: Zacks Investment Research
Other Stocks to Consider
Here are some more drug/biotech stocks that you may want to consider, as our model shows that they have the right combination of elements to post an earnings beat this season.
Akero Therapeutics AKRO has an Earnings ESP of +6.45% and a Zacks Rank #2. You can see the complete list of today’s Zacks #1 Rank stocks here.
AKRO beat earnings estimate in three of the last four quarters and missed in the remaining one, the average earnings surprise being 8.38%.
Acadia ACAD has an Earnings ESP of +14.65% and a Zacks Rank #3.
ACAD topped earnings estimates in two of the last four quarters and missed in the remaining two. It has a four-quarter negative earnings surprise of 6.33%, on average.
Stay on top of upcoming earnings announcements with the Zacks Earnings Calendar.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Regeneron Pharmaceuticals, Inc. (REGN) : Free Stock Analysis Report
Sanofi (SNY) : Free Stock Analysis Report
ACADIA Pharmaceuticals Inc. (ACAD) : Free Stock Analysis Report
Akero Therapeutics, Inc. (AKRO) : Free Stock Analysis Report

 Yahoo Finance
Yahoo Finance 