AbbVie's (ABBV) Rinvoq Meets Goals in Crohn's Disease Study
AbbVie ABBV announced positive top-line results from a late-stage study, U-EXCEED, on Rinvoq (upadacitinib), a selective and reversible JAK inhibitor.
AbbVie’s study enrolled patients with moderate-to-severe Crohn's disease who had an inadequate response or were intolerant to biologic therapy, with over 60% having previously failed two or more biologics.
In the U-EXCEED study, a significantly higher proportion of patients with moderate-to-severe Crohn's disease treated with Rinvoq (45 mg once daily for induction) achieved both primary endpoints of clinical remission and endoscopic response compared to placebo at week 12. Clinical remission was measured by the Crohn's Disease Activity Index (CDAI) and by the patient-reported symptoms of stool frequency/abdominal pain (SF/AP).
U-EXCEED is the first of the two phase III induction studies to evaluate the safety and efficacy of Rinvoq in adults with moderate-to-severe Crohn's disease.
Among patients taking corticosteroids at baseline, a significantly higher proportion receiving Rinvoq 45 mg achieved steroid-free clinical remission per CDAI and per SF/AP compared to placebo at week 12.
We note that Rinvoq 15 mg is approved in the United States to treat moderate-to-severe rheumatoid arthritis in adults when one or more tumor necrosis factor (TNF) blockers have been used and did not work well or could not be tolerated. It is also approved by the European Commission for adults (15 mg and 30 mg) and adolescents (15 mg) with moderate-to-severe atopic dermatitis. Rinvoq 15 mg is approved by the European Commission for adults with moderate-to-severe active rheumatoid arthritis in adults with active psoriatic arthritis and adults with active ankylosing spondylitis.
Phase III studies in rheumatoid arthritis, atopic dermatitis, psoriatic arthritis, axial spondyloarthritis, Crohn's disease, ulcerative colitis, giant cell arteritis and Takayasu arteritis are currently ongoing.
A potential label expansion of the drug will increase the sales potential and reduce AbbVie’s dependence on its legacy drug, Humira.
AbbVie’s stock has risen 13.3% this year so far compared with an increase of 12.8% for the industry.
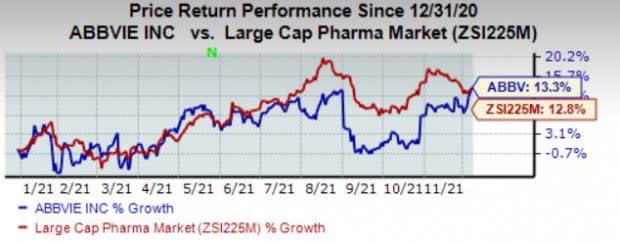
Image Source: Zacks Investment Research
However, AbbVie recently announced an update to the prescribing information for Rinvoq for the treatment of adults with moderate-to-severe rheumatoid arthritis.
The update follows a Drug Safety Communication (DSC) issued in September by the FDA following its final review of the post-marketing study evaluating Pfizer’s PFE Xeljanz (tofacitinib) in patients with RA.
The results of this study showed a higher rate of major adverse cardiac events (MACE), malignancy, mortality and thrombosis in Pfizer’s Xeljanz (a Janus kinase [JAK] inhibitor) versus TNF blockers. The DSC and this label update apply to the class of systemically administered FDA-approved JAKs indicated for the treatment of RA and other inflammatory diseases.
Consequently, the updated Rinvoq label includes boxed warnings about an increased risk of serious heart-related events, cancer, blood clots, and even death. This might have a limiting impact on sales.
AbbVie currently has a Zacks Rank #3 (Hold). A couple of better-ranked stocks in the healthcare sector are Sarepta Therapeutics, Inc. SRPT and Viking Therapeutics VKTX, both carrying a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Estimates for Sarepta have narrowed from a loss per share of $6.95 to $4.99 for 2021 and from $4.83 to $3.61 for 2022 in the past 30 days. SRPT delivered an earnings surprise of 11.06%, on average, in the last four quarters.
Estimates for Viking Therapeutics have narrowed to a loss per share of $3.16 from $3.55 for 2021 and to $3.47 from $3.63 for 2022 in the past 30 days. VKTX delivered an earnings surprise of 2.06%, on average, in the last four quarters.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Sarepta Therapeutics, Inc. (SRPT) : Free Stock Analysis Report
AbbVie Inc. (ABBV) : Free Stock Analysis Report
Viking Therapeutics, Inc. (VKTX) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research

 Yahoo Finance
Yahoo Finance 